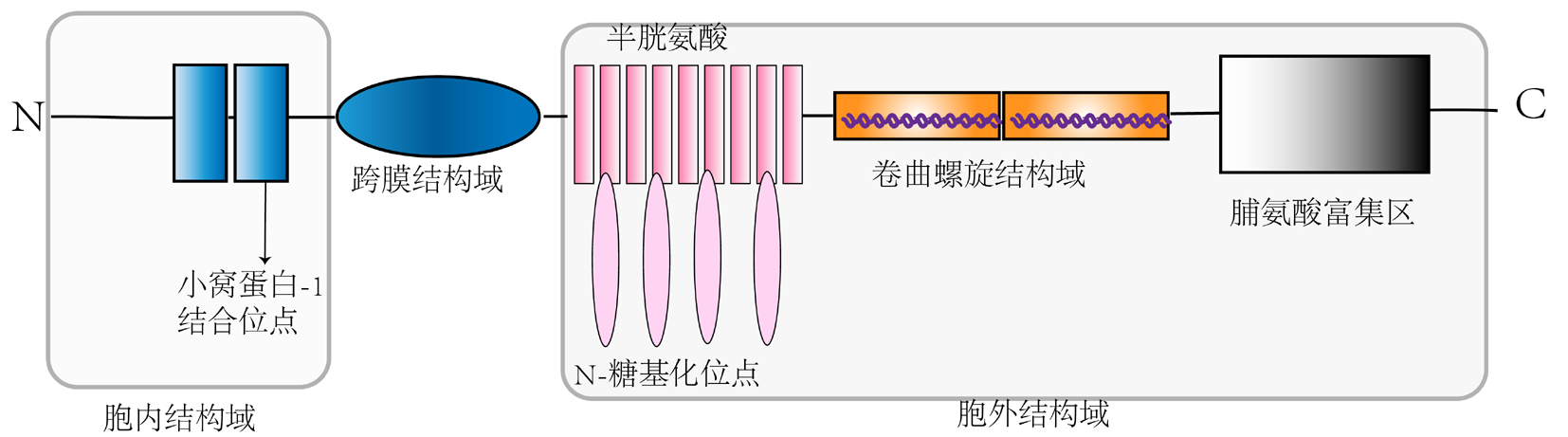
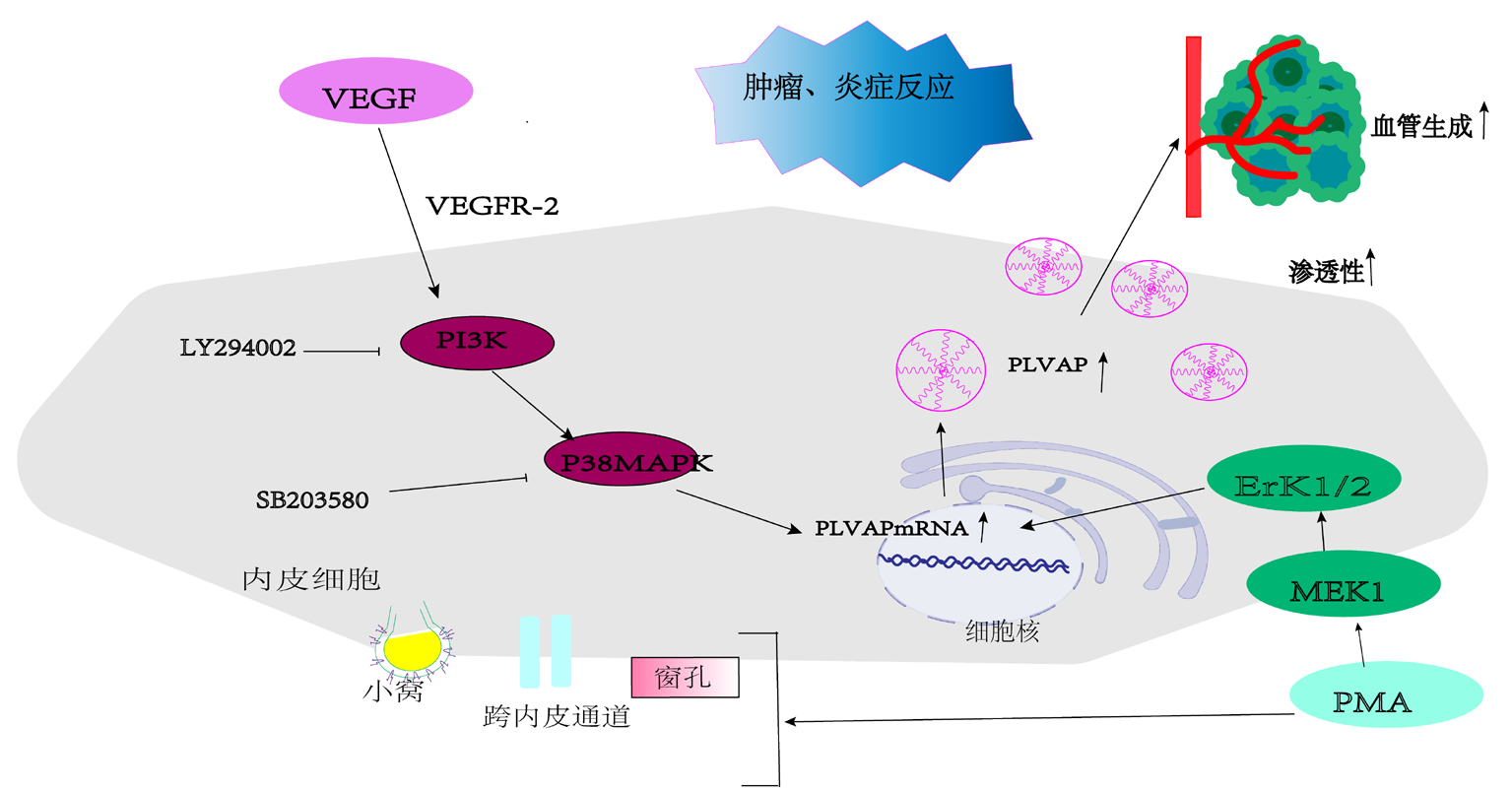
Clinical Focus ›› 2023, Vol. 38 ›› Issue (7): 647-653.doi: 10.3969/j.issn.1004-583X.2023.07.011
Previous Articles Next Articles
-
Received:2023-03-18Online:2023-07-20Published:2023-09-01
CLC Number:
Cite this article
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2023.07.011
| 疾病 | PLVAP水平 | 可能的机制 |
|---|---|---|
| 癌症[ | 升高 | 血管生成、通透性增加 |
| 急性脑缺血病[ | 升高 | 血管生成、通透性增加 |
| 脂肪型肝炎[ | 降低 | 窗孔调节、基底膜形成 |
| 蛋白丢失性肠病[ | 突变 | 渗透性增加 |
| 糖尿病性视网膜病变[ | 升高 | 血管生成、通透性增加 |
| 疾病 | PLVAP水平 | 可能的机制 |
|---|---|---|
| 癌症[ | 升高 | 血管生成、通透性增加 |
| 急性脑缺血病[ | 升高 | 血管生成、通透性增加 |
| 脂肪型肝炎[ | 降低 | 窗孔调节、基底膜形成 |
| 蛋白丢失性肠病[ | 突变 | 渗透性增加 |
| 糖尿病性视网膜病变[ | 升高 | 血管生成、通透性增加 |
| 疾病 | 手段 | 病理机制 |
|---|---|---|
| 肝癌[ | 重组单克隆抗PLVAP Fab-TF | 抗血管生成 |
| 胆管癌[ | 增加内皮细胞血管 生成 | |
| 胰腺癌[ | shRAN PLVAP | 抗血管生成 |
| 恶性胶质瘤[ | 抗血管生成、免疫细胞的浸润 | |
| 糖尿病视网膜 病变[ | 靶向VEGFA-PLVAP | 抗血管生成、保护血视网膜屏障 |
| 肺部疾病[ | 脂质纳米粒 | 维持血管完整性 |
| 病毒性脑炎[ | PLVAP基因沉默 | 控制日本脑炎病毒在神经元的繁殖 |
| 疾病 | 手段 | 病理机制 |
|---|---|---|
| 肝癌[ | 重组单克隆抗PLVAP Fab-TF | 抗血管生成 |
| 胆管癌[ | 增加内皮细胞血管 生成 | |
| 胰腺癌[ | shRAN PLVAP | 抗血管生成 |
| 恶性胶质瘤[ | 抗血管生成、免疫细胞的浸润 | |
| 糖尿病视网膜 病变[ | 靶向VEGFA-PLVAP | 抗血管生成、保护血视网膜屏障 |
| 肺部疾病[ | 脂质纳米粒 | 维持血管完整性 |
| 病毒性脑炎[ | PLVAP基因沉默 | 控制日本脑炎病毒在神经元的繁殖 |
| [1] |
Eelen G, Treps L, Li X, et al. Basic and therapeutic aspects of angiogenesis updated[J]. Circ Res, 2020, 127(2):310-329.
doi: 10.1161/CIRCRESAHA.120.316851 pmid: 32833569 |
| [2] |
Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells[J]. Nature, 2016, 529(7586):316-325.
doi: 10.1038/nature17040 |
| [3] |
Nourshargh S, Alon R. Leukocyte migration into inflamed tissues[J]. Immunity, 2014, 41(5):694-707.
doi: 10.1016/j.immuni.2014.10.008 pmid: 25517612 |
| [4] |
Stan RV. Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis[J]. J Cell Mol Med, 2007, 11(4):621-643.
doi: 10.1111/j.1582-4934.2007.00075.x pmid: 17760829 |
| [5] |
Stan RV, Tse D, Deharvengt SJ, et al. The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition[J]. Dev Cell, 2012, 23(6):1203-1218.
doi: 10.1016/j.devcel.2012.11.003 pmid: 23237953 |
| [6] |
Herrnberger L, Seitz R, Kuespert S, et al. Lack of endothelial diaphragms in fenestrae and caveolae of mutant Plvap-deficient mice[J]. Histochem Cell Biol, 2012, 138(5):709-724.
doi: 10.1007/s00418-012-0987-3 pmid: 22782339 |
| [7] |
Rantakari P, Auvinen K, Jäppinen N, et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes[J]. Nat Immunol, 2015, 16(4):386-396.
doi: 10.1038/ni.3101 pmid: 25665101 |
| [8] |
Liu Y, Carson-Walter EB, Cooper A, et al. Vascular gene expression patterns are conserved in primary and metastatic brain tumors[J]. J Neurooncol, 2010, 99(1):13-24.
doi: 10.1007/s11060-009-0105-0 URL |
| [9] |
Keuschnigg J, Henttinen T, Auvinen K, et al. The prototype endothelial marker PAL-E is a leukocyte trafficking molecule[J]. Blood, 2009, 114(2): 478-484.
doi: 10.1182/blood-2008-11-188763 pmid: 19420356 |
| [10] | Minshall RD, Malik AB. Transport across the endothelium: Regulation of endothelial permeability[J]. Handb Exp Pharmacol, 2006, (176 Pt 1):107-144. |
| [11] | Wen Y, Wang Y, Huang Y, et al. PLVAP protein expression correlated with microbial composition, clinicopathological features, and prognosis of patients with stomach adenocarcinoma[J]. J Cancer Res Clin Oncol, 2023, 99(1): 13-24. |
| [12] |
Terkelsen MK, Bendixen SM, Hansen D, et al. Transcriptional dynamics of hepatic sinusoid-associated cells after liver injury[J]. Hepatology, 2020, 72(6): 2119-2133.
doi: 10.1002/hep.31215 URL |
| [13] |
Gorukmez O, Gorukmez O, Demiroren K. Novel PLVAP mutation in protein losing enteropathy[J]. Fetal Pediatr Pathol, 2019, 38(6):534-537.
doi: 10.1080/15513815.2019.1627624 pmid: 31215290 |
| [14] |
Laksitorini MD, Yathindranath V, Xiong W, et al. Impact of Wnt/β-catenin signaling on ethanol-induced changes in brain endothelial cell permeability[J]. J Neurochem, 2021, 157(4):1118-1137.
doi: 10.1111/jnc.v157.4 URL |
| [15] |
Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia[J]. Proc Natl Acad Sci U S A, 1999, 96(23):13203-13207.
doi: 10.1073/pnas.96.23.13203 URL |
| [16] |
Ghitescu LD, Crine P, Jacobson BS. Antibodies specific to the plasma membrane of rat lung microvascular endothelium[J]. Exp Cell Res, 1997, 232(1):47-55.
pmid: 9141620 |
| [17] |
Stan RV. Structure of caveolae[J]. Biochim Biophys Acta, 2005, 1746(3):334-348.
pmid: 16214243 |
| [18] |
Hnasko R, Ben-Jonathan N. Developmental regulation of PV-1 in rat lung: Association with the nuclear envelope and limited colocalization with Cav-1[J]. Am J Physiol Lung Cell Mol Physiol, 2005, 288(2):L275-284.
doi: 10.1152/ajplung.00236.2004 URL |
| [19] |
Stan RV, Ghitescu L, Jacobson BS, et al. Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein[J]. J Cell Biol, 1999, 145(6):1189-1198.
pmid: 10366592 |
| [20] |
Strickland LA, Jubb AM, Hongo JA, et al. Plasmalemmal vesicle-associated protein (PLVAP) is expressed by tumour endothelium and is upregulated by vascular endothelial growth factor-A (VEGF)[J]. J Pathol, 2005, 206(4): 466-475.
pmid: 15971170 |
| [21] |
Hofman P, Blaauwgeers HG, Vrensen GF, et al. Role of VEGF-A in endothelial phenotypic shift in human diabetic retinopathy and VEGF-A-induced retinopathy in monkeys[J]. Ophthalmic Res, 2001, 33(3):156-162.
pmid: 11340407 |
| [22] |
Hnasko R, Frank P, Ben-Jonathan N, et al. PV-1 is negatively regulated by VEGF in the lung of caveolin-1, but not caveolin-2, null mice[J]. Cell cycle (Georgetown, Tex), 2006, 5(17):2012-2020.
doi: 10.4161/cc.5.17.3216 URL |
| [23] |
Denzer L, Muranyi W, Schroten H, et al. The role of PLVAP in endothelial cells[J]. Cell Tissue Res., 2023, 392(2): 393-412.
doi: 10.1007/s00441-023-03741-1 |
| [24] |
Hamilton BJ, Tse D, Stan RV. Phorbol esters induce PLVAP expression via VEGF and additional secreted molecules in MEK1-dependent and p38, JNK and PI3K/Akt-independent manner[J]. J Cell Mol Med, 2019, 23(2):920-933.
doi: 10.1111/jcmm.13993 pmid: 30394679 |
| [25] |
He L, Lu H, Ji X, et al. Stimulatory G-protein α subunit modulates endothelial cell permeability through regulation of plasmalemma vesicle-associated protein[J]. Front Pharmacol, 2022, 13:941064.
doi: 10.3389/fphar.2022.941064 URL |
| [26] |
Bodor C, Nagy JP, Végh B, et al. Angiotensin II increases the permeability and PV-1 expression of endothelial cells[J]. Am J Physiol Cell Physiol, 2012, 302(1):C267-276.
doi: 10.1152/ajpcell.00138.2011 URL |
| [27] |
Farber G, Hurtado R, Loh S, et al. Glomerular endothelial cell maturation depends on ADAM10, a key regulator of Notch signaling[J]. Angiogenesis, 2018, 21(2):335-347.
doi: 10.1007/s10456-018-9599-4 pmid: 29397483 |
| [28] |
Wasserman SM, Mehraban F, Komuves LG, et al. Gene expression profile of human endothelial cells exposed to sustained fluid shear stress[J]. Physiol Genomics, 2002, 12(1):13-23.
doi: 10.1152/physiolgenomics.00102.2002 pmid: 12419857 |
| [29] |
Stan RV, Tkachenko E, Niesman IR. PV1 is a key structural component for the formation of the stomatal and fenestral diaphragms[J]. Mol Biol Cell, 2004, 15(8): 3615-3630.
pmid: 15155804 |
| [30] |
Ioannidou S, Deinhardt K, Miotla J, et al. An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis[J]. Proc Natl Acad Sci U S A, 2006, 103(45):16770-16775.
doi: 10.1073/pnas.0603501103 URL |
| [31] |
Zhao Y, Zhao J. PV1: Gatekeeper of endothelial permeability[J]. Am J Respir Cell Mol Biol, 2020, 63(4):413-414.
doi: 10.1165/rcmb.2020-0294ED URL |
| [32] |
Jones JH, Friedrich E, Hong Z, et al. PV1 in caveolae controls lung endothelial permeability[J]. Am J Respir Cell Mol Biol, 2020, 63(4):531-539.
doi: 10.1165/rcmb.2020-0102OC URL |
| [33] |
Elgueta R, Tse D, Deharvengt SJ, et al. Endothelial plasmalemma vesicle-associated protein regulates the homeostasis of splenic immature B cells and B-1 B cells[J]. J Immunol, 2016, 197(10):3970-3981.
pmid: 27742829 |
| [34] |
Madden SL, Cook BP, Nacht M, et al. Vascular gene expression in nonneoplastic and malignant brain[J]. Am J Pathol, 2004, 165(2):601-608.
pmid: 15277233 |
| [35] |
Carson-Walter EB, Hampton J, Shue E, et al. Plasmalemmal vesicle associated protein-1 is a novel marker implicated in brain tumor angiogenesis[J]. Clin Cancer Res, 2005, 11(21):7643-7650.
pmid: 16278383 |
| [36] |
Ma K, Chen X, Zhao X, et al. PLVAP is associated with glioma-associated malignant processes and immunosuppressive cell infiltration as a promising marker for prognosis[J]. Heliyon, 2022, 8(8):e10298.
doi: 10.1016/j.heliyon.2022.e10298 URL |
| [37] |
Wang Y, Yu H, Xie X, et al. Plasmalemma vesicle-associated protein promotes angiogenesis in cholangiocarcinoma via the DKK1/CKAP4/PI3K signaling pathway[J]. Oncogene, 2021, 40(25):4324-4337.
doi: 10.1038/s41388-021-01844-z pmid: 34079085 |
| [38] | 熊志勇, 姚志成, 胡昆鹏, 等. PLVAP基因在肝癌组织的表达及临床意义[J]. 中华肝脏外科手术学电子杂志, 2018, 7(6):511-515. |
| [39] |
Bertocchi A, Carloni S, Ravenda PS, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver[J]. Cancer Cell, 2021, 39(5):708-724.
doi: 10.1016/j.ccell.2021.03.004 pmid: 33798472 |
| [40] |
Deharvengt SJ, Tse D, Sideleva O, et al. PV1 down-regulation via shRNA inhibits the growth of pancreatic adenocarcinoma xenografts[J]. J Cell Mol Med, 2012, 16(11):2690-2700.
doi: 10.1111/j.1582-4934.2012.01587.x pmid: 22568538 |
| [41] |
Tichauer KM, Deharvengt SJ, Samkoe KS, et al. Tumor endothelial marker imaging in melanomas using dual-tracer fluorescence molecular imaging[J]. Mol Imaging Biol, 2014, 16(3): 372-382.
doi: 10.1007/s11307-013-0692-1 pmid: 24217944 |
| [42] |
Boyé K, Geraldo LH, Furtado J, et al. Endothelial Unc5B controls blood-brain barrier integrity[J]. Nat Commun, 2022, 13(1):1169.
doi: 10.1038/s41467-022-28785-9 pmid: 35246514 |
| [43] |
Shue EH, Carson-Walter EB, Liu Y, et al. Plasmalemmal vesicle associated protein-1 (PV-1) is a marker of blood-brain barrier disruption in rodent models[J]. BMC Neurosci, 2008, 9:29.
doi: 10.1186/1471-2202-9-29 pmid: 18302779 |
| [44] |
Herrnberger L, Hennig R, Kremer W, et al. Formation of fenestrae in murine liver sinusoids depends on plasmalemma vesicle-associated protein and is required for lipoprotein passage[J]. PLoS One, 2014, 9(12):e115005.
doi: 10.1371/journal.pone.0115005 URL |
| [45] |
Desroches-Castan A, Tillet E, Ricard N, et al. Bone morphogenetic protein 9 is a paracrine factor controlling liver sinusoidal endothelial cell fenestration and protecting against hepatic fibrosis[J]. Hepatology, 2019, 70(4):1392-1408.
doi: 10.1002/hep.30655 pmid: 30964206 |
| [46] |
Elkadri A, Thoeni C, Deharvengt SJ, et al. Mutations in plasmalemma vesicle associated protein result in sieving protein-losing enteropathy characterized by hypoproteinemia, hypoalbuminemia, and hypertriglyceridemia[J]. Cell Mol Gastroenterol Hepatol, 2015, 1(4):381-394.
pmid: 26207260 |
| [47] |
Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic ma cular edema and other pathological conditions[J]. Prog Retin Eye Res, 2013, 34:19-48.
doi: 10.1016/j.preteyeres.2013.02.001 URL |
| [48] | Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes[J]. Invest Ophthalmol Vis Sci, 2001, 42(10):2408-2413. |
| [49] |
Witmer AN, Vrensen GF, Van Noorden CJ, et al. Vascular endothelial growth factors and angiogenesis in eye disease[J]. Prog Retin Eye Res, 2003, 22(1):1-29.
doi: 10.1016/s1350-9462(02)00043-5 pmid: 12597922 |
| [50] |
Klaassen I, Hughes JM, Vogels IM, et al. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes[J]. Exp Eye Res, 2009, 89(1):4-15.
doi: 10.1016/j.exer.2009.01.006 pmid: 19284967 |
| [51] |
Wisniewska-Kruk J, Klaassen I, Vogels IM, et al. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy[J]. Exp Eye Res, 2014, 122:123-131.
doi: 10.1016/j.exer.2014.03.005 pmid: 24703908 |
| [52] |
Wisniewska-Kruk J, van der Wijk AE, van Veen HA, et al. Plasmalemma vesicle-associated protein has a key role in blood-retinal barrier loss[J]. Am J Pathol, 2016, 186(4):1044-1054.
doi: 10.1016/j.ajpath.2015.11.019 pmid: 26878208 |
| [53] |
Wang Y, Cheng T, Chen T, et al. Plasmalemmal vesicle associated protein (PLVAP) as a therapeutic target for treatment of hepatocellular carcinoma[J]. BMC cancer, 2014, 14:815.
doi: 10.1186/1471-2407-14-815 URL |
| [54] |
Bosma EK, Schlingemann RO, et al. The role of plasmalemma vesicle-associated protein in pathological breakdown of blood-brain and blood-retinal barriers: Potential novel therapeutic target for cerebral edema and diabetic macular edema[J]. Fluids Barriers Cns, 2018, 15(1):24.
doi: 10.1186/s12987-018-0109-2 pmid: 30231925 |
| [55] |
Li Q, Chan C, Peterson N, et al. Engineering caveolae-targeted lipid nanoparticles to deliver mRNA to the lungs[J]. ACS Chem Biol, 2020, 15(4):830-836.
doi: 10.1021/acschembio.0c00003 pmid: 32155049 |
| [56] |
Marchetti GM, Burwell TJ, Peterson NC et al. Targeted drug delivery via caveolae-associated protein PV1 improves lung fibrosis[J]. Commun Biol, 2019, 2: 92.
doi: 10.1038/s42003-019-0337-2 pmid: 30854484 |
| [57] |
Mukherjee S, Sengupta N, Chaudhuri A, et al. PLVAP and GKN3 are two critical host cell receptors which facilitate Japanese encephalitis virus entry into neurons[J]. Sci Rep, 2018, 8(1):11784.
doi: 10.1038/s41598-018-30054-z pmid: 30082709 |
| [1] | . [J]. Clinical Focus, 2023, 38(11): 1053-1056. |
| [2] | . [J]. Clinical Focus, 2023, 38(8): 763-768. |
| [3] | Ye Qian, Ling Zhai, Liu Shenxiang, Lu Guotao, Yin Xudong. Meta analysis on effects of glucocorticoid on the immunotherapy of advanced cancer [J]. Clinical Focus, 2022, 37(7): 591-598. |
| [4] | Zhou Yixuan, Lin Weizhao, Li Ruiman. Relationship between homocysteine and hypertensive disorder complicating pregnancy: a meta-analysis [J]. Clinical Focus, 2021, 36(2): 101-106. |
| [5] | Deng Yujie, Fan Linqing, Zhang Tao. Relations between lipoprotein-associated phospholipase A2 and unstable carotid atherosclerotic plaque in middle-aged or elderly patients with hypertension [J]. Clinical Focus, 2021, 36(2): 112-116. |
| [6] | Qian Zhengyao, Li Guangping, Li Jiao, Liang Xue, Xu Zhao, Chen Yan, Zhao Hui. Relationship between PCSK9 and inflammation factor expression in ox-LDL-induced endothelial cells apoptosis [J]. Clinical Focus, 2016, 31(4): 396-402. |
| [7] | . [J]. Clinical Focus, 2014, 29(11): 1294-1296. |
| [8] | YAO Xiao-ling;LIU Jian-jun;YANG Xiu-hua;WANG Jie-chao;LIU Wei-lin;CHANG Cui-fen;SHANG Su-liang. Ginkgo biloba extract on apoptosis of endothelial cells [J]. Clinical Focus, 2014, 29(9): 1022-1024. |
| [9] | . [J]. Clinical Focus, 2014, 29(9): 1078-1081. |
| [10] | TANG Ying;LIU Wei-qun;REN Jian-ru;WU Yang. Relationship between dyslipidemia and thyrotropin with reference range in euthyroid men [J]. CLINICAL FOCUS, 2014, 29(4): 389-391. |
| [11] | CHEN Lin;YU Ming. Effects of caffeine on proliferation and apoptosis of β cells exposed to palmitic acid in vitro [J]. CLINICAL FOCUS, 2013, 28(7): 772-774782. |
| [12] | . [J]. CLINICAL FOCUS, 2013, 28(7): 783-785. |
| [13] | . [J]. Clinical Focus, 2012, 27(3): 270-272. |
| [14] | ZHANG Hui-min;HUANG Peng;LIU Li;PENG Chun-xia;PENG Ding-qiong;LU Bing-huai. Relationship between changes of serum thyroid hormone levels and prognosis of geriatric critical disease [J]. CLINICAL FOCUS, 2011, 26(3): 212-214. |
| [15] | LONG Li;SHAN Ke-ren;ZHAO Yan;LI Yi;HE Yan;WU Chang-xue;QI Xiao-lan;XIE Yuan;ZHANG Ting;GUAN Zhi-zhong;REN Xi-lin. Investigation on association of estrogen receptor α polymorphism with susceptibility to hepatitis B virus infection in Han nationality and Yao minority from Guizhou province [J]. CLINICAL FOCUS, 2009, 24(15): 1305-1308. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||