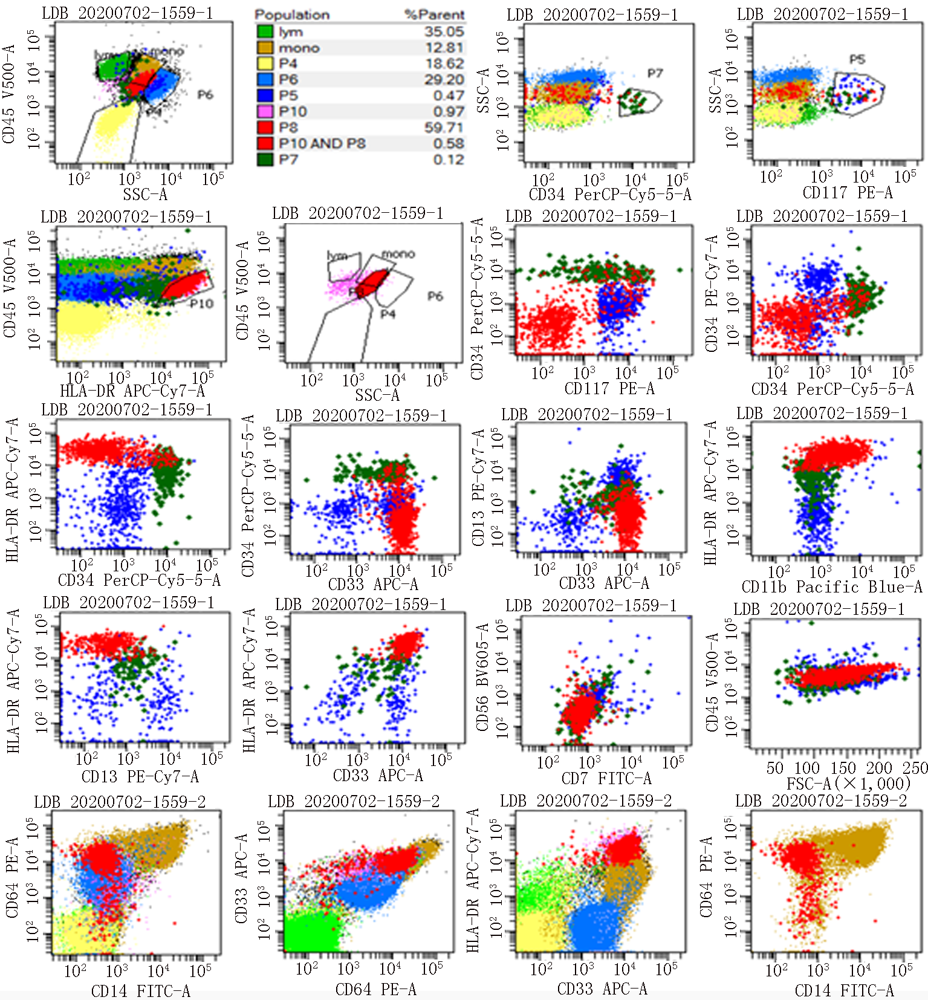
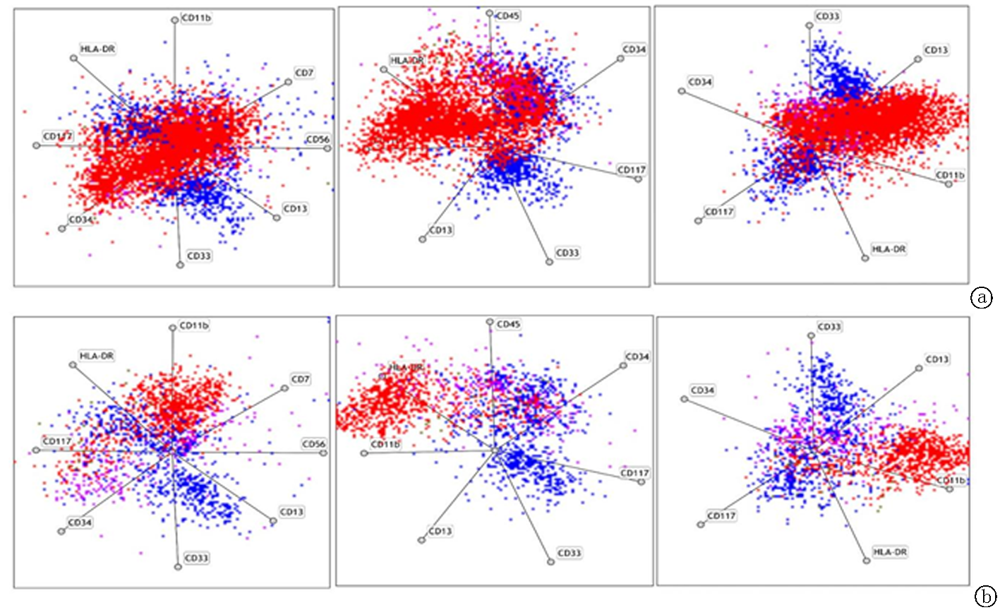
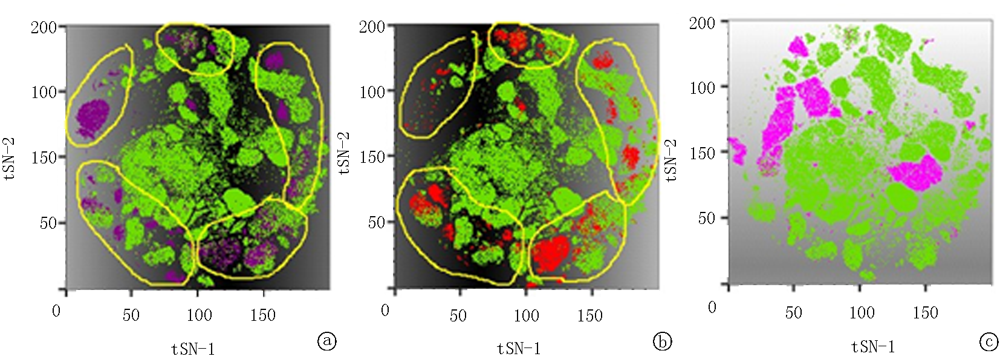
Clinical Focus ›› 2021, Vol. 36 ›› Issue (10): 889-895.doi: 10.3969/j.issn.1004-583X.2021.10.005
Previous Articles Next Articles
Flow cytometry in minimal residual disease detection of acute myeloid leukemia: current status and progress
- Clinical Laboratory, Hebei Yanda Ludaopei Hospital, Sanhe 065201, China
-
Received:2021-07-16Online:2021-10-20Published:2021-11-10 -
Contact:Wang Hui E-mail:wh9784@163.com
CLC Number:
Cite this article
Wang Hui, Chen Man. Flow cytometry in minimal residual disease detection of acute myeloid leukemia: current status and progress[J]. Clinical Focus, 2021, 36(10): 889-895.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2021.10.005
| 抗原性质 | 标志 | 发生率 |
|---|---|---|
| 原始细胞/骨架标志 | CD45 CD34 CD117 HLA-DR和(或)CD33 | |
| 伴系表达标志 | CD4 | ≥50% |
| CD7▼ CD9# | ≥30%~<50% | |
| CD56△ CD25* | ≥10%~<30% | |
| CD19△ CD2▲ CD5 CD10 | <10% | |
| 过早获得的成熟阶段标志 | CD18▽ | ≥50% |
| CD15▼ CD65 CD36 CD24 | ≥30%~<50% | |
| CD11b CD64▽ CD11c | ≥10%~<30% | |
| CD14▼↓ | <10% | |
| 表达强度改变标志 | CD81 | ≥50% |
| (包括丢失、获得) | CD96△↑ CD123#↑ CD371(CLL1)△↑ CD200△ CD366(tim3)△↑ | ≥30%~<50% |
| CD38 CD33▼↑ CD13#↑▼↑ CD34#↓ CD117#↑ HLA-DR#↓▼↑ CD184(CXCR4) | ≥10%~<30% | |
| 其他可选标志 | CD244 CD52△↑ CD97 CD54▲↑ CD170 CD300f GPR56 CD11a★↓ | ≥50% |
| CD59△↓ CD44 CD300a/c△↓ CX3CR1 CD86 CD32 CD1c CD26 CD66c CD273(PDL2) | ≥30%~<50% | |
| CD47 CD99 CD68▽↑ CD93▲↑ | ≥10%~<30% | |
| 肿瘤干细胞标志 | CD9 CD69 CD93 CD371 IL-1RAP* CD114 CD135 CD123 | ≥50% |
| CD25*# CD26*# CD96 CD116 CD332 | ≥30%~<50% | |
| CD115 CD221 | ≥10%~<30% |
| 抗原性质 | 标志 | 发生率 |
|---|---|---|
| 原始细胞/骨架标志 | CD45 CD34 CD117 HLA-DR和(或)CD33 | |
| 伴系表达标志 | CD4 | ≥50% |
| CD7▼ CD9# | ≥30%~<50% | |
| CD56△ CD25* | ≥10%~<30% | |
| CD19△ CD2▲ CD5 CD10 | <10% | |
| 过早获得的成熟阶段标志 | CD18▽ | ≥50% |
| CD15▼ CD65 CD36 CD24 | ≥30%~<50% | |
| CD11b CD64▽ CD11c | ≥10%~<30% | |
| CD14▼↓ | <10% | |
| 表达强度改变标志 | CD81 | ≥50% |
| (包括丢失、获得) | CD96△↑ CD123#↑ CD371(CLL1)△↑ CD200△ CD366(tim3)△↑ | ≥30%~<50% |
| CD38 CD33▼↑ CD13#↑▼↑ CD34#↓ CD117#↑ HLA-DR#↓▼↑ CD184(CXCR4) | ≥10%~<30% | |
| 其他可选标志 | CD244 CD52△↑ CD97 CD54▲↑ CD170 CD300f GPR56 CD11a★↓ | ≥50% |
| CD59△↓ CD44 CD300a/c△↓ CX3CR1 CD86 CD32 CD1c CD26 CD66c CD273(PDL2) | ≥30%~<50% | |
| CD47 CD99 CD68▽↑ CD93▲↑ | ≥10%~<30% | |
| 肿瘤干细胞标志 | CD9 CD69 CD93 CD371 IL-1RAP* CD114 CD135 CD123 | ≥50% |
| CD25*# CD26*# CD96 CD116 CD332 | ≥30%~<50% | |
| CD115 CD221 | ≥10%~<30% |
| 荧光素方案 | 标本 (管) | FITC | PE | PerCP- Cy5.5或 PE-Cy5 | PE-CY7 | APC | APC-H7 或其他 | BV421或 深蓝或 V450 | V500或 深黄 | BV605 | APC R700 或APC- Alexa Fluor700 | PE-TR | A594 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 中国免疫学会流式细胞学组[ | 全 | CD38 | X* | CD33 | CD34 | CD13 | HLA-DR | CD117 | CD45 | X* | X* | ||
| 华盛顿大学[ | 1 | CD15 | CD33 | CD117 | CD13 | CD34 | CD45 | HLA-DR | CD71 | CD19 | CD38 | ||
| 2 | CD64 | CD123 | CD14 | CD13 | CD34 | CD45 | HLA-DR | CD16 | CD4 | CD38 | |||
| 3 | CD56 | CD7 | CD5 | CD33 | CD34 | CD45 | CD38 | ||||||
| MD Anderson[ | 1 | CD7 | CD33 | CD19 | CD34 | CD13 | CD38 | CD45 | |||||
| 2 | HLA-DR | CD117 | CD4 | CD34 | CD123 | CD19 eF780 | CD38 | CD45 | |||||
| 3 | HLA-DR | CD36 | CD56 | CD34 | CD64 | CD19 eF780 | CD14 | CD45 | |||||
| 4 | CD5 | CD2 | CD22 | CD34 | CD38 | CD19 eF780 | CD15 | CD45 | |||||
| 荷兰瑞士协作组[ | 白血病干细胞 | CD45RA | CD371△ | CD123 | CD34 | CD38 | CD44 | CD33 | CD45 | ||||
| 1 | CD7 | CD56 | CD34 | CD117 | CD33 | HLA-DR | CD13 | CD45 | |||||
| 2 | CD15 | CD22 | CD34 | CD117 | CD19 | HLA-DR | CD13 | CD45 | |||||
| 3 | CD36 | CD14 | CD34 | CD117 | CD11b | HLA-DR | CD13 | CD45 | |||||
| 4# | CD2 | CD133 | CD34 | CD117 | CD33 | HLA-DR | CD13 | CD45 | |||||
| 陆道培医院1▲ | 1 | CD7 | CD117 | CD34 | CD13 | CD33 | HLA-DR | CD11b | CD45 | CD56 | |||
| 2 | CD15 | CD64 | CD34 | CD117 | CD14 | HLA-DR | CD19 | CD45 | |||||
| 陆道培医院2▽ | 1 | CD33 | CD96 | CD34 | CD13 | CD117 | HLA-DR | CD11b | CD45 | ||||
| 2 | CD7 | CD117 | CD34 | CD19 | CD56 | CD14 | CD38 | CD45 |
| 荧光素方案 | 标本 (管) | FITC | PE | PerCP- Cy5.5或 PE-Cy5 | PE-CY7 | APC | APC-H7 或其他 | BV421或 深蓝或 V450 | V500或 深黄 | BV605 | APC R700 或APC- Alexa Fluor700 | PE-TR | A594 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 中国免疫学会流式细胞学组[ | 全 | CD38 | X* | CD33 | CD34 | CD13 | HLA-DR | CD117 | CD45 | X* | X* | ||
| 华盛顿大学[ | 1 | CD15 | CD33 | CD117 | CD13 | CD34 | CD45 | HLA-DR | CD71 | CD19 | CD38 | ||
| 2 | CD64 | CD123 | CD14 | CD13 | CD34 | CD45 | HLA-DR | CD16 | CD4 | CD38 | |||
| 3 | CD56 | CD7 | CD5 | CD33 | CD34 | CD45 | CD38 | ||||||
| MD Anderson[ | 1 | CD7 | CD33 | CD19 | CD34 | CD13 | CD38 | CD45 | |||||
| 2 | HLA-DR | CD117 | CD4 | CD34 | CD123 | CD19 eF780 | CD38 | CD45 | |||||
| 3 | HLA-DR | CD36 | CD56 | CD34 | CD64 | CD19 eF780 | CD14 | CD45 | |||||
| 4 | CD5 | CD2 | CD22 | CD34 | CD38 | CD19 eF780 | CD15 | CD45 | |||||
| 荷兰瑞士协作组[ | 白血病干细胞 | CD45RA | CD371△ | CD123 | CD34 | CD38 | CD44 | CD33 | CD45 | ||||
| 1 | CD7 | CD56 | CD34 | CD117 | CD33 | HLA-DR | CD13 | CD45 | |||||
| 2 | CD15 | CD22 | CD34 | CD117 | CD19 | HLA-DR | CD13 | CD45 | |||||
| 3 | CD36 | CD14 | CD34 | CD117 | CD11b | HLA-DR | CD13 | CD45 | |||||
| 4# | CD2 | CD133 | CD34 | CD117 | CD33 | HLA-DR | CD13 | CD45 | |||||
| 陆道培医院1▲ | 1 | CD7 | CD117 | CD34 | CD13 | CD33 | HLA-DR | CD11b | CD45 | CD56 | |||
| 2 | CD15 | CD64 | CD34 | CD117 | CD14 | HLA-DR | CD19 | CD45 | |||||
| 陆道培医院2▽ | 1 | CD33 | CD96 | CD34 | CD13 | CD117 | HLA-DR | CD11b | CD45 | ||||
| 2 | CD7 | CD117 | CD34 | CD19 | CD56 | CD14 | CD38 | CD45 |
| 特点 | 传统流式细胞术 | 二代流式细胞术 | 全光谱流式细胞术 | 质谱流式细胞术 |
|---|---|---|---|---|
| 原理 | 多采用光电倍增管 | 同传统流式 | 使用光电倍增管或者雪崩二极管,多个探测器检测一个荧光素形成连续光谱 | 使用各种金属元素作为标签;使用质谱技术作为检测手段 |
| 激光 | 1~3 | 3激光 | 3~5激光 | 使用质谱,非激光 |
| 荧光素(色) | 4~13 | ≥8 | 理论上38~184通道,目前抗体限制检测24~40色 | 理论可以检测130多种蛋白,目前受标志物限制,检测50多个蛋白或者RNA |
| 获取细胞数 | 105~106 | 5×106~2×107 | 同传统流式 | 几百万细胞中进行单细胞分析 |
| 灵敏度 | 10-3~10-4 | 10-5~10-6 | 同传统流式 | 速度限制(获取1000个/s),目前灵敏度有限 |
| 操作 | 多数先标记后溶血 | 建议增加样本量,先溶血后标记 | 多数同传统流式 | 金属标签标记 |
| 方案 | 个体化 | 标准化 | 个体化 | 个体化 |
| 分析方法 | 差别大,多数人工 | 相对固定,人工+半自动 | 人工 | 人工 |
| 软件要求 | 多使用Diva、Kaluza等二维参数分析软件,极少使用高维软件进行分析。 | 可以使用Diva、Kaluza等常规软件,建议使用Infinicyt配套软件。除了常规平面图,使用多维自动群聚分析图进行多参数群聚分析。 | 除了Spectroflo二维软件,一般会使用Flowjo、Cytobank等多维软件及插件进行多维和群聚分析。 | 一般会使用Flowjo、Cytobank等多维软件及插件进行多维和群聚分析。 |
| 优点 | 临床适用性最高:操作简单,速度快,分析简单,相互补偿干扰小,数据容量小,一般电脑满足需求。 | 灵敏度最高:本质是对传统高端流式进行操作和分析的规范化。操作简单,标本量少的实验室一般电脑可以满足需求。 | 性价比最高:可以实现多参数检测,做好单阳管质控,可以减除自发荧光。 | 技术高度最高:可以同时进行上百个独立通道检测, 且通道间无干扰,无需补偿。除了免疫表型分析,还能检测胞内信号通路等深入研究。 |
| 缺点 | 检测参数少,灵敏度较低。 | 耗时长,数据占用空间大,对电脑容量和内存要求高。 | 需要找到合适的单阳管做解析,数据分析复杂,对软件和电脑要求高。 | 获取速度慢,成本高,分析复杂,需要高端电脑和软件。 |
| 特点 | 传统流式细胞术 | 二代流式细胞术 | 全光谱流式细胞术 | 质谱流式细胞术 |
|---|---|---|---|---|
| 原理 | 多采用光电倍增管 | 同传统流式 | 使用光电倍增管或者雪崩二极管,多个探测器检测一个荧光素形成连续光谱 | 使用各种金属元素作为标签;使用质谱技术作为检测手段 |
| 激光 | 1~3 | 3激光 | 3~5激光 | 使用质谱,非激光 |
| 荧光素(色) | 4~13 | ≥8 | 理论上38~184通道,目前抗体限制检测24~40色 | 理论可以检测130多种蛋白,目前受标志物限制,检测50多个蛋白或者RNA |
| 获取细胞数 | 105~106 | 5×106~2×107 | 同传统流式 | 几百万细胞中进行单细胞分析 |
| 灵敏度 | 10-3~10-4 | 10-5~10-6 | 同传统流式 | 速度限制(获取1000个/s),目前灵敏度有限 |
| 操作 | 多数先标记后溶血 | 建议增加样本量,先溶血后标记 | 多数同传统流式 | 金属标签标记 |
| 方案 | 个体化 | 标准化 | 个体化 | 个体化 |
| 分析方法 | 差别大,多数人工 | 相对固定,人工+半自动 | 人工 | 人工 |
| 软件要求 | 多使用Diva、Kaluza等二维参数分析软件,极少使用高维软件进行分析。 | 可以使用Diva、Kaluza等常规软件,建议使用Infinicyt配套软件。除了常规平面图,使用多维自动群聚分析图进行多参数群聚分析。 | 除了Spectroflo二维软件,一般会使用Flowjo、Cytobank等多维软件及插件进行多维和群聚分析。 | 一般会使用Flowjo、Cytobank等多维软件及插件进行多维和群聚分析。 |
| 优点 | 临床适用性最高:操作简单,速度快,分析简单,相互补偿干扰小,数据容量小,一般电脑满足需求。 | 灵敏度最高:本质是对传统高端流式进行操作和分析的规范化。操作简单,标本量少的实验室一般电脑可以满足需求。 | 性价比最高:可以实现多参数检测,做好单阳管质控,可以减除自发荧光。 | 技术高度最高:可以同时进行上百个独立通道检测, 且通道间无干扰,无需补偿。除了免疫表型分析,还能检测胞内信号通路等深入研究。 |
| 缺点 | 检测参数少,灵敏度较低。 | 耗时长,数据占用空间大,对电脑容量和内存要求高。 | 需要找到合适的单阳管做解析,数据分析复杂,对软件和电脑要求高。 | 获取速度慢,成本高,分析复杂,需要高端电脑和软件。 |
| [1] |
Del Principe MI, Buccisano F, Soddu S, et al. Involvement of central nervous system in adult patients with acute myeloid leukemia: Incidence and impact on outcome[J]. Semin Hematol, 2018,55(4):209-214.
doi: S0037-1963(17)30104-X pmid: 30502849 |
| [2] | Araki D, Wood BL, Othus M, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission?[J]. J Clin Oncol, 2016,34(4):329-336. |
| [3] | 卢岳, 吴彤, 王卉 等. 预处理前多参数流式细胞术监测的微小残留病对急性髓系白血病异基因造血干细胞移植预后的影响[J]. 中华血液学杂志, 2017,38(2):118-123. |
| [4] |
Getta BM, Devlin SM, Levine RL, et al. Multicolor flow cytometry and multigene next-generation sequencing are complementary and highly predictive for relapse in acute myeloid leukemia after allogeneic transplantation[J]. Biol Blood Marrow Transplant, 2017,23(7):1064-1071.
doi: 10.1016/j.bbmt.2017.03.017 URL |
| [5] | 常英军, 赵晓甦. 多参数流式细胞仪检测急性髓细胞白血病微小残留病:挑战与对策[J]. 临床荟萃, 2016,31(6):581-584. |
| [6] |
Kriegsmann K, Löffler H, Eckstein V, et al. CD7 is expressed on a subset of normal CD34-positive myeloid precursors[J]. Eur J Haematol, 2018,101(3):318-325.
doi: 10.1111/ejh.13100 pmid: 29797671 |
| [7] |
Camburn AE, Petrasich M, Ruskova A, et al. Myeloblasts in normal bone marrows expressing leukaemia-associated immunophenotypes[J]. Pathology, 2019,51(5):502-506.
doi: 10.1016/j.pathol.2019.03.010 URL |
| [8] | Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group[J]. Blood, 2012,120(8):1581-1588. |
| [9] | 中国免疫学会血液免疫分会临床流式细胞术学组. 多参数流式细胞术检测急性白血病及浆细胞肿瘤微小残留病中国专家共识(2017年版)[J]. 中华血液学杂志, 2017,38(12):1001-1011. |
| [10] |
Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party[J]. Blood, 2018,131(12):1275-1291.
doi: 10.1182/blood-2017-09-801498 pmid: 29330221 |
| [11] |
Wang Z, Guo M, Zhang Y, et al. The applicability of multiparameter flow cytometry for the detection of minimal residual disease using different-from-normal panels to predict relapse in patients with acute myeloid leukemia after allogeneic transplantation[J]. Int J Lab Hematol, 2019,41(5):607-614.
doi: 10.1111/ijlh.v41.5 URL |
| [12] |
Zhou Y, Moon A, Hoyle E, et al. Pattern associated leukemia immunophenotypes and measurable disease detection in acute myeloid leukemia or myelodysplastic syndrome with mutated NPM1[J]. Cytometry B Clin Cytom, 2019,96(1):67-72.
doi: 10.1002/cyto.b.v96.1 URL |
| [13] |
Ouyang J, Goswami M, Peng J, et al. Comparison of multiparameter flow cytometry immunophenotypic analysis and quantitative RT-PCR for the detection of minimal residual disease of core binding factor acute myeloid leukemia[J]. Am J Clin Pathol, 2016,145(6):769-777.
doi: 10.1093/ajcp/aqw038 pmid: 27298396 |
| [14] | Zeijlemaker W, Kelder A, Cloos J, et al. Immunophenotypic detection of measurable residual (stem cell) disease using LAIP approach in acute myeloid leukemia[J]. Curr Protoc Cytom, 2019,91(1):e66. |
| [15] |
Brodersen LE, Gerbing RB, Pardo ML, et al. Morphologic remission status is limited compared to ΔN flow cytometry: A Children's Oncology Group AAML0531 report[J]. Blood Adv, 2020,4(20):5050-5061.
doi: 10.1182/bloodadvances.2020002070 URL |
| [16] | Cloos J, Harris JR, Janssen JJWM, et al. Comprehensive protocol to sample and process bone marrow for measuring measurable residual disease and leukemic stem cells in acute myeloid leukemia[J]. J Vis Exp, 2018,133:56386. |
| [17] | Wood BL. Acute myeloid leukemia minimal residual disease detection: The difference from normal approach[J]. Curr Protoc Cytom, 2020,93(1):e73. |
| [18] | Bento LC, Correia RP, Alexandre AM, et al. Detection of central nervous system Infiltration by myeloid and lymphoid hematologic neoplasms using flow cytometry analysis: Diagnostic accuracy study[J]. Front Med (Lausanne), 2018,5:70. |
| [19] | Langebrake C, Brinkmann I, Teigler-Schlegel A, et al. Immunophenotypic differences between diagnosis and relapse in childhood AML: Implications for MRD monitoring[J]. Cytometry B Clin Cytom, 2005,63(1):1-9. |
| [20] |
Voskova D, Schoch C, Schnittger S, et al. Stability of leukemia-associated aberrant immunophenotypes in patients with acute myeloid leukemia between diagnosis and relapse: Comparison with cytomorphologic, cytogenetic, and molecular genetic findings[J]. Cytometry B Clin Cytom, 2004,62B:25-38.
doi: 10.1002/(ISSN)1097-0320 URL |
| [21] |
Daga S, Rosenberger A, Quehenberger F, et al. High GPR56 surface expression correlates with a leukemic stem cell gene signature in CD34-positive AML[J]. Cancer Med, 2019,8(4):1771-1778.
doi: 10.1002/cam4.2019.8.issue-4 URL |
| [22] |
Herrmann H, Sadovnik I, Eisenwort G, et al. Delineation of target expression profiles in CD34+/CD38- and CD34+/CD38+ stem and progenitor cells in AML and CML[J]. Blood Adv, 2020,4(20):5118-5132.
doi: 10.1182/bloodadvances.2020001742 pmid: 33085758 |
| [23] |
Heo SK, Noh EK, Ju LJ, et al. CD45dimCD34+CD38-CD133+ cells have the potential as leukemic stem cells in acute myeloid leukemia[J]. BMC Cancer, 2020,20(1):285.
doi: 10.1186/s12885-020-06760-1 URL |
| [24] |
Coustan-Smith E, Song G, Shurtleff S, et al. Universal monitoring of minimal residual disease in acute myeloid leukemia[J]. JCI Insight, 2018,3(9):e98561.
doi: 10.1172/jci.insight.98561 URL |
| [25] | Gjertsen B, Tislevoll B, Eagerholt O, et al. Response evaluation in AML using mass cytometry[J]. Hema Sphere, 2019,3:S2. |
| [26] |
Niewold P, Ashhurst TM, Smith AL, et al. Evaluating spectral cytometry for immune profiling in viral disease[J]. Cytometry A, 2020,97(11):1165-1179.
doi: 10.1002/cyto.a.v97.11 URL |
| [27] |
Vial JP, Lechevalier N, Lacombe F, et al. Unsupervised flow cytometry analysis allows for an accurate identification of minimal residual disease assessment in acute myeloid leukemia[J]. Cancers (Basel), 2021,13(4):629.
doi: 10.3390/cancers13040629 URL |
| [28] | Jacqmin H, Chatelain B, Louveaux Q, et al. Clustering and kernel density estimation for assessment of measurable residual disease by flow cytometry[J]. Diagnostics (Basel), 2020,10(5):317. |
| [29] |
Jiang L, Li XP, Dai YT, et al. Multidimensional study of the heterogeneity of leukemia cells in t(8;21) acute myelogenous leukemia identifies the subtype with poor outcome[J]. Proc Natl Acad Sci USA, 2020,117(33):20117-20126.
doi: 10.1073/pnas.2003900117 URL |
| [1] | Lu Wenyi, Zhao Mingfeng. The standard treatment of acute lymphoblastic leukemia [J]. Clinical Focus, 2021, 36(10): 874-879. |
| [2] | Ren Jinhai, Guo Xiaonan. Clinical advances on neoplastic haematologic disorders in 2017 [J]. Clinical Focus, 2018, 33(1): 40-45. |
| [3] | Yang Lina. Expression and significance of neutrophil surface adhesion molecules CD11b from cytomegalovirus infection in children [J]. Clinical Focus, 2017, 32(8): 703-706. |
| [4] | Chen Yongquan, Yang Ting. Current treatment strategies for refractory/relapsed Hodgkin's lymphoma [J]. Clinical Focus, 2017, 32(12): 1022-1026. |
| [5] | Chang Yingjun1,2, Zhao Xiaosu2. Detection of minimal residual disease using flow cytometry in patients with acute myeloid leukemia: challenges and tactics [J]. Clinical Focus, 2016, 31(6): 581-584. |
| [6] | Luo Hong;Chen Yanfei. Expression of interleukin-1β in platelet of patients with rheumatoid arthritis [J]. Clinical Focus, 2015, 30(8): 903-905. |
| [7] | Ren Jinhai;Guo Xiaonan;Lin Fengru. Advance in acute myeloid leukemia therapy 2014 [J]. Clinical Focus, 2015, 30(2): 157-160. |
| [8] | LI Zhen;FANG Bai-jun. Advances in treatment of chronic myelogenous leukemia [J]. Clinical Focus, 2014, 29(10): 1126-1.12911e+007. |
| [9] | CUI Jiu-wei. Treatments of chronic lymphocytic leukemia [J]. Clinical Focus, 2014, 29(10): 1119-1125. |
| [10] | GUO Xiao-ling. Updates in treatment of relapsed and refractory acute myeloid leukemia [J]. Clinical Focus, 2014, 29(10): 1108-1113. |
| [11] | WEI Hui. Treatment of acute lymphoblastic leukemia [J]. Clinical Focus, 2014, 29(10): 1100-1103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||