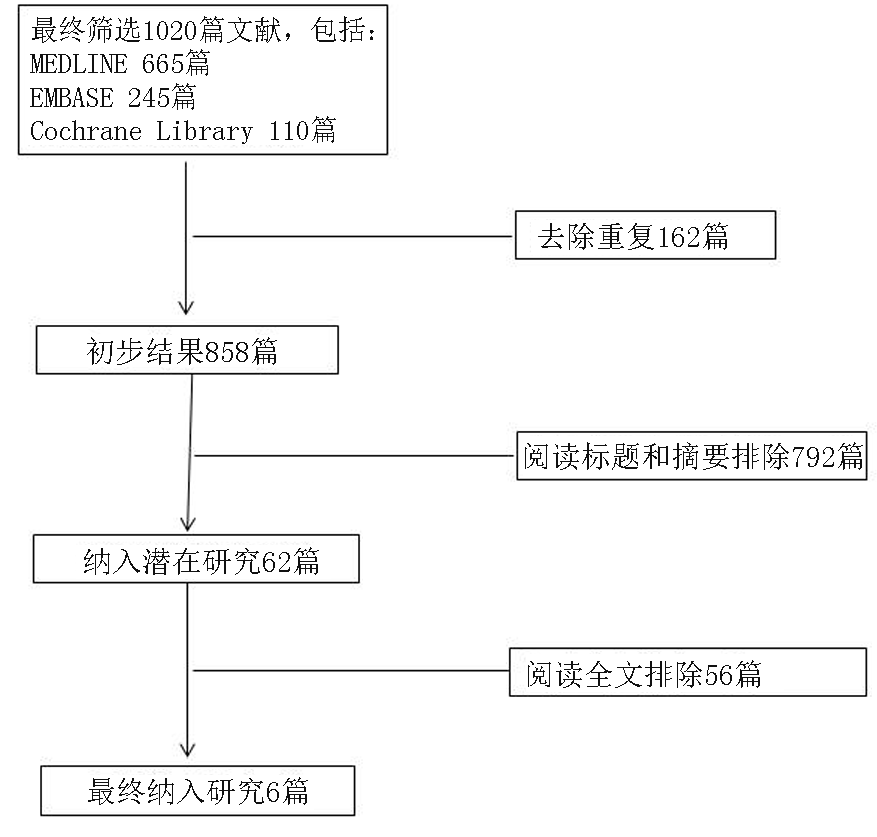
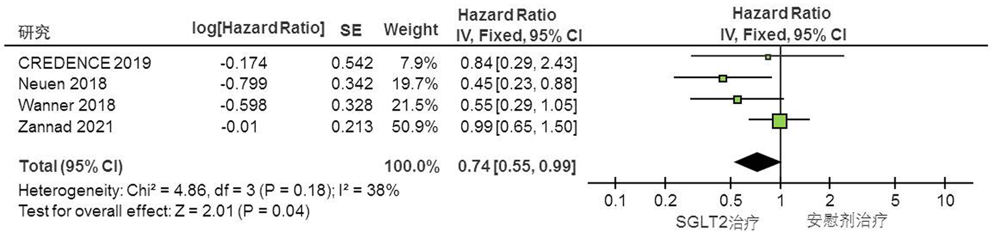
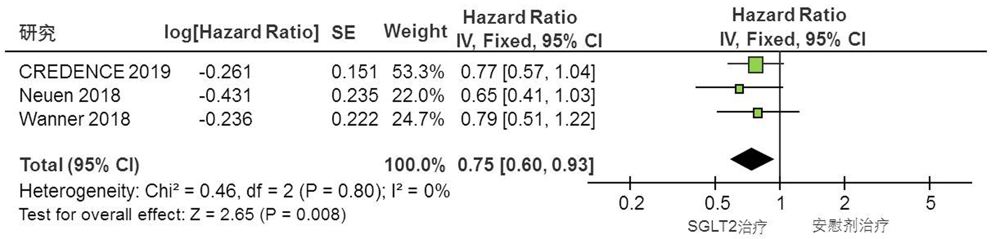
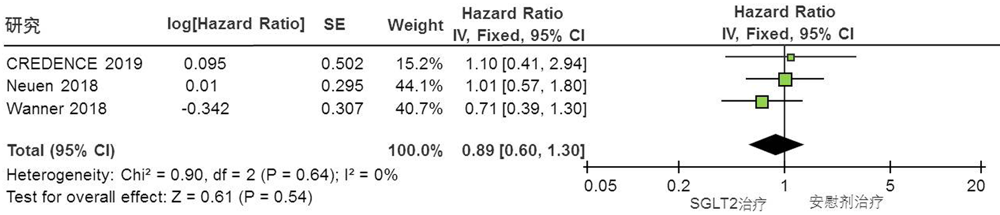
Clinical Focus ›› 2022, Vol. 37 ›› Issue (1): 14-19.doi: 10.3969/j.issn.1004-583X.2022.01.002
Previous Articles Next Articles
Cardiovascular protective effects of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus patients with severe renal insufficiency: A meta-analysis
Xu Cangdan( ), Zhao Xin, Gu Wenyuan
), Zhao Xin, Gu Wenyuan
- Department of Endocrinology, Second Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300150, China
-
Received:2021-09-07Online:2022-01-20Published:2022-01-20 -
Contact:Xu Cangdan E-mail:xucangdan@163.com
CLC Number:
Cite this article
Xu Cangdan, Zhao Xin, Gu Wenyuan. Cardiovascular protective effects of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus patients with severe renal insufficiency: A meta-analysis[J]. Clinical Focus, 2022, 37(1): 14-19.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2022.01.002
| 纳入研究 | 治疗时间 (周) | 药物 | 平均年龄 (岁) | T2DM确诊时间 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | ||||||||||||||||
| Barnett 2014 | 52 | 恩格列净(25 mg) | 安慰剂 | 65.4(10.2) | 62.9(11.9) | 15年:10.8%; 510年:16.2%; >10年:73.0% | 15年:16.2% 510年:21.6% >10年:62.2% | ||||||||||||||
| CREDENCE 2019 | 136 | 卡格列净(100 mg) | 安慰剂 | 62.9(9.2) | 63.2(9.2) | 15.5年 | 16.0年 | ||||||||||||||
| Neuen 2018 | 188.2 | 卡格列净 (100 mg,300 mg) | 安慰剂 | 68.7(8.0) | - | - | |||||||||||||||
| Wanner 2018 | 52 | 恩格列净 (10 mg,25 mg) | 安慰剂 | 66.2(8.0) | 66.0(8.5) | <1年:2.1%; 15年:10.1% 510年:21.9%; >10年:66.0% | <1年:1.2%; 15年:9.9%; 510年:19.8%; >10年:68.9% | ||||||||||||||
| Yale 2014 | 52 | 卡格列净 (100 mg,300 mg) | 安慰剂 | 卡格列净100 mg:69.5(8.2) 卡格列净300 mg:67.9 (8.2) | 68.2(8.4) | 卡格列净100 mg:15.6年 卡格列净300 mg:17.0年 | 16.4年 | ||||||||||||||
| Zannad 2021 | 16 | 恩格列净(10 mg) | 安慰剂 | 70.4(9.5) | 70.1(9.8) | / | / | ||||||||||||||
| 纳入研究 | 体重(kg) | eGFR [ml/(min·1.73 m2)] | HbA1c(%) | 样本量(例) | Jadad 评分 | ||||||||||||||||
| 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | ||||||||||||||
| Barnett 2014 | 77.9±16.4 | 84.1±21.1 | 1530 | 1530 | 7.96 | 8.08 | 37 | 37 | 4 | ||||||||||||
| CREDENCE 2019 | / | / | 3045 | 3045 | 8.3 | 8.3 | 657 | 656 | 4 | ||||||||||||
| Neuen 2018 | - | - | 3045 | 3045 | - | - | 554 | 4 | |||||||||||||
| Wanner 2018 | 86.84±11.01 | 85.49±11.43 | 3045 | 3045 | 8.07 | 8.08 | 381 | 189 | |||||||||||||
| Yale 2014 | 卡格列净100 mg:90.5±18.4 卡格列净300 mg:90.2±18.1 | 92.8±17.4 | 3050 | 3050 | 卡格列净100 mg:7.9 卡格列净300 mg:8 | 8 | 卡格列净100 mg:90 卡格列净300 mg:89 | 90 | 4 | ||||||||||||
| Zannad 2021 | - | - | 1545 | 1545 | / | / | 460 | 439 | 4 | ||||||||||||
| 纳入研究 | 治疗时间 (周) | 药物 | 平均年龄 (岁) | T2DM确诊时间 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | ||||||||||||||||
| Barnett 2014 | 52 | 恩格列净(25 mg) | 安慰剂 | 65.4(10.2) | 62.9(11.9) | 15年:10.8%; 510年:16.2%; >10年:73.0% | 15年:16.2% 510年:21.6% >10年:62.2% | ||||||||||||||
| CREDENCE 2019 | 136 | 卡格列净(100 mg) | 安慰剂 | 62.9(9.2) | 63.2(9.2) | 15.5年 | 16.0年 | ||||||||||||||
| Neuen 2018 | 188.2 | 卡格列净 (100 mg,300 mg) | 安慰剂 | 68.7(8.0) | - | - | |||||||||||||||
| Wanner 2018 | 52 | 恩格列净 (10 mg,25 mg) | 安慰剂 | 66.2(8.0) | 66.0(8.5) | <1年:2.1%; 15年:10.1% 510年:21.9%; >10年:66.0% | <1年:1.2%; 15年:9.9%; 510年:19.8%; >10年:68.9% | ||||||||||||||
| Yale 2014 | 52 | 卡格列净 (100 mg,300 mg) | 安慰剂 | 卡格列净100 mg:69.5(8.2) 卡格列净300 mg:67.9 (8.2) | 68.2(8.4) | 卡格列净100 mg:15.6年 卡格列净300 mg:17.0年 | 16.4年 | ||||||||||||||
| Zannad 2021 | 16 | 恩格列净(10 mg) | 安慰剂 | 70.4(9.5) | 70.1(9.8) | / | / | ||||||||||||||
| 纳入研究 | 体重(kg) | eGFR [ml/(min·1.73 m2)] | HbA1c(%) | 样本量(例) | Jadad 评分 | ||||||||||||||||
| 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | ||||||||||||||
| Barnett 2014 | 77.9±16.4 | 84.1±21.1 | 1530 | 1530 | 7.96 | 8.08 | 37 | 37 | 4 | ||||||||||||
| CREDENCE 2019 | / | / | 3045 | 3045 | 8.3 | 8.3 | 657 | 656 | 4 | ||||||||||||
| Neuen 2018 | - | - | 3045 | 3045 | - | - | 554 | 4 | |||||||||||||
| Wanner 2018 | 86.84±11.01 | 85.49±11.43 | 3045 | 3045 | 8.07 | 8.08 | 381 | 189 | |||||||||||||
| Yale 2014 | 卡格列净100 mg:90.5±18.4 卡格列净300 mg:90.2±18.1 | 92.8±17.4 | 3050 | 3050 | 卡格列净100 mg:7.9 卡格列净300 mg:8 | 8 | 卡格列净100 mg:90 卡格列净300 mg:89 | 90 | 4 | ||||||||||||
| Zannad 2021 | - | - | 1545 | 1545 | / | / | 460 | 439 | 4 | ||||||||||||
| 不良反应 | 报道研究数 | SGLT2抑制剂 | 安慰剂 | 异质性检验 | 统计学方法 | RR值 | P值 | 95%CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 例数 | 总例数 | 例数 | 总例数 | P值 | I2(%) | 下限 | 上限 | |||||||
| 总不良事件 | 3 | 260 | 300 | 190 | 217 | 0.4 | 0 | M-H, Fixed | 1 | 0.96 | 0.94 | 1.07 | ||
| 骨折 | 2 | 10 | 274 | 2 | 128 | 0.54 | 0 | M-H, Fixed | 2.34 | 0.27 | 0.52 | 10.51 | ||
| 泌尿道感染 | 2 | 25 | 216 | 12 | 127 | 0.48 | 0 | M-H, Fixed | 1.22 | 0.54 | 0.64 | 2.35 | ||
| 低血压 | 2 | 18 | 216 | 8 | 127 | 0.6 | 0 | M-H, Fixed | 1.32 | 0.49 | 0.59 | 2.95 | ||
| 生殖道感染 | 1 | 4 | 179 | 3 | 90 | - | - | M-H, Fixed | 0.67 | 0.6 | 0.15 | 2.93 | ||
| 急性肾损伤 | 1 | 9 | 84 | 10 | 90 | - | - | M-H, Fixed | 0.96 | 0.93 | 0.41 | 2.26 | ||
| 不良反应 | 报道研究数 | SGLT2抑制剂 | 安慰剂 | 异质性检验 | 统计学方法 | RR值 | P值 | 95%CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 例数 | 总例数 | 例数 | 总例数 | P值 | I2(%) | 下限 | 上限 | |||||||
| 总不良事件 | 3 | 260 | 300 | 190 | 217 | 0.4 | 0 | M-H, Fixed | 1 | 0.96 | 0.94 | 1.07 | ||
| 骨折 | 2 | 10 | 274 | 2 | 128 | 0.54 | 0 | M-H, Fixed | 2.34 | 0.27 | 0.52 | 10.51 | ||
| 泌尿道感染 | 2 | 25 | 216 | 12 | 127 | 0.48 | 0 | M-H, Fixed | 1.22 | 0.54 | 0.64 | 2.35 | ||
| 低血压 | 2 | 18 | 216 | 8 | 127 | 0.6 | 0 | M-H, Fixed | 1.32 | 0.49 | 0.59 | 2.95 | ||
| 生殖道感染 | 1 | 4 | 179 | 3 | 90 | - | - | M-H, Fixed | 0.67 | 0.6 | 0.15 | 2.93 | ||
| 急性肾损伤 | 1 | 9 | 84 | 10 | 90 | - | - | M-H, Fixed | 0.96 | 0.93 | 0.41 | 2.26 | ||
| [1] | Galbete A, Cambra K, Forga L, et al. Achievement of cardiovascular risk factor targets according to sex and previous history of cardiovascular disease in type 2 diabetes: A population-based study[J]. J Diabetes Complications, 2019, 33(12):107445. |
| [2] |
American Diabetes Association. Standards of medical care in diabetes-2020 abridged for primary care providers[J]. Clin Diabetes, 2020, 38(1):10-38.
doi: 10.2337/cd20-as01 URL |
| [3] |
Wright AK, Suarez-Ortegon MF, Read SH, et al. Risk factor control and cardiovascular event risk in people with type 2 diabetes in primary and secondary prevention settings[J]. Circulation, 2020, 142(20):1925-1936.
doi: 10.1161/CIRCULATIONAHA.120.046783 URL |
| [4] | Saisho Y. SGLT2 Inhibitors: the star in the treatment of type 2 diabetes?[J]. Diseases, 2020, 8(2). |
| [5] | Fala L. Jardiance (Empagliflozin), an SGLT2 inhibitor, receives FDA approval for the treatment of patients with type 2 diabetes[J]. Am Health Drug Benefits, 2015, 8(Spec Feature):92-95. |
| [6] |
Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: A state-of-the-art review[J]. JACC Basic Transl Sci, 2020, 5(6):632-644.
doi: 10.1016/j.jacbts.2020.02.004 pmid: 32613148 |
| [7] |
Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials[J]. Lancet, 2020, 396(10254):819-829.
doi: S0140-6736(20)31824-9 pmid: 32877652 |
| [8] |
Cowie MR, Fisher M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control[J]. Nat Rev Cardiol, 2020, 17(12):761-772.
doi: 10.1038/s41569-020-0406-8 URL |
| [9] |
Nespoux J, Vallon V. Renal effects of SGLT2 inhibitors:An update[J]. Curr Opin Nephrol Hypertens, 2020, 29(2):190-198.
doi: 10.1097/MNH.0000000000000584 URL |
| [10] |
Tsimihodimos V, Filippatos TD, Elisaf MS. SGLT2 inhibitors and the kidney: Effects and mechanisms[J]. Diabetes Metab Syndr, 2018, 12(6):1117-1123.
doi: S1871-4021(18)30208-X pmid: 29909004 |
| [11] |
Clark HD, Wells GA, Huet C, et al. Assessing the quality of randomized trials: Reliability of the Jadad scale[J]. Control Clin Trials, 1999, 20(5):448-452.
doi: 10.1016/s0197-2456(99)00026-4 pmid: 10503804 |
| [12] |
Zannad F, Ferreira JP, Pocock SJ, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: Insights from emperor-reduced[J]. Circulation, 2021, 143(4):310-321.
doi: 10.1161/CIRCULATIONAHA.120.051685 pmid: 33095032 |
| [13] |
Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function[J]. Circulation, 2018, 138(15):1537-1550.
doi: 10.1161/CIRCULATIONAHA.118.035901 URL |
| [14] |
Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease[J]. Diabetes Obes Metab, 2014, 16(10):1016-1027.
doi: 10.1111/dom.12348 pmid: 24965700 |
| [15] |
Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial[J]. Lancet Diabetes Endocrinol, 2014, 2(5):369-384.
doi: 10.1016/S2213-8587(13)70208-0 URL |
| [16] |
Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease[J]. Circulation, 2018, 137(2):119-129.
doi: 10.1161/CIRCULATIONAHA.117.028268 URL |
| [17] |
Rizzo MR, Barbieri M, Grella R, et al. Repaglinide has more beneficial effect on cardiovascular risk factors than glimepiride: Data from meal-test study[J]. Diabetes Metab, 2005, 31(3 Pt 1):255-260.
pmid: 16142016 |
| [18] |
Nakagawa Y, Kuwahara K. Sodium-glucose cotransporter-2 inhibitors are potential therapeutic agents for treatment of non-diabetic heart failure patients[J]. J Cardiol, 2020, 76(2):123-131.
doi: S0914-5087(20)30122-2 pmid: 32340780 |
| [19] |
Vardeny O. The sweet spot: Heart failure prevention with SGLT2 inhibitors[J]. Am J Med, 2020, 133(2):182-185.
doi: S0002-9343(19)30706-5 pmid: 31494110 |
| [20] |
Zhou Z, Jardine MJ, Li Q, et al. Effect of SGLT2 inhibitors on stroke and atrial fibrillation in diabetic kidney disease: Results from the credence trial and meta-analysis[J]. Stroke, 2021, 52(5):1545-1556.
doi: 10.1161/STROKEAHA.120.031623 URL |
| [21] | Bell D, Goncalves E. Stroke in the patient with diabetes (Part 2) - Prevention and the effects of glucose lowering therapies[J]. Diabetes Res Clin Pract, 2020, 164:108199. |
| [22] | Rocha NA, Mccullough PA. Cardiovascular outcomes in diabetic kidney disease: Insights from recent clinical trials[J]. Kidney Int Suppl (2011), 2018, 8(1):8-17. |
| [23] | Gajos G. Diabetes and cardiovascular disease: From new mechanisms to new therapies[J]. Pol Arch Intern Med, 2018, 128(3):178-186. |
| [24] | Oelze M, Kroller-Schon S, Welschof P, et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity[J]. PLoS One, 2014, 9(11):e112394. |
| [25] |
Joshi SS, Singh T, Newby DE, et al. Sodium-glucose co-transporter 2 inhibitor therapy: Mechanisms of action in heart failure[J]. Heart, 2021 107(13):1032-1038.
doi: 10.1136/heartjnl-2020-318060 URL |
| [26] |
Lunder M, Janic M, Japelj M, et al. Empagliflozin on top of metformin treatment improves arterial function in patients with type 1 diabetes mellitus[J]. Cardiovasc Diabetol, 2018, 17(1):153.
doi: 10.1186/s12933-018-0797-6 pmid: 30509271 |
| [27] |
Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease[J]. Nat Commun, 2020, 11(1):2127.
doi: 10.1038/s41467-020-15983-6 pmid: 32358544 |
| [28] |
Maejima Y. SGLT2 inhibitors play a salutary role in heart failure via modulation of the mitochondrial function[J]. Front Cardiovasc Med, 2019, 6:186.
doi: 10.3389/fcvm.2019.00186 URL |
| [29] |
Esteban-Jimenez O, Navarro-Peman C, Urieta-Gonzalez L. Safety of SGLT2 inhibitors. A review of the adverse drug reactions registered in a national database[J]. Semergen, 2018, 44(1):23-29.
doi: S1138-3593(17)30302-7 pmid: 29183654 |
| [30] |
Bai Y, Jin J, Zhou W, et al. The safety outcomes of sodium-glucose cotransporter 2 inhibitors in patients with different renal function: A systematic review and meta-analysis[J]. Nutr Metab Cardiovasc Dis, 2021, 31(5):1365-1374.
doi: 10.1016/j.numecd.2021.02.006 URL |
| [1] | Dong Hui, Huang Wenhui, Zhao Hui, Qian Rui. Adult Bartter syndrome complicated with acute exacerbation of chronic renal insufficiency: A case report [J]. Clinical Focus, 2023, 38(11): 1022-1026. |
| [2] | Liu Xiangdong, Cai Yandong, Qin Yanjun, Li Yunsong, Li Liang, Gao Ruijiao, Ren Lei, Zhang Yanrong. Exertional rhabdomyolysis complicated with acute renal insufficiency and catheter-related thrombosis: One case and literature review [J]. Clinical Focus, 2022, 37(9): 831-833. |
| [3] | Wang Lingling, Wu Jinlan, Wu Wei, Shen Shipeng, Chi Yanqing, Liu Maodong. A meta-analysis of the renal protective effects of vitamin D and its analogues in non dialysis patients with chronic kidney disease [J]. Clinical Focus, 2022, 37(12): 1081-1088. |
| [4] | Zheng Junxia, Ou Jinghua. Predictive value of uKIM-1 and uL-FABP levels on early renal function injury in patients with pregnancy-induced hypertension syndrome [J]. Clinical Focus, 2021, 36(12): 1102-1105. |
| [5] | Cheng Mingrong, Dai Dejian. Diagnostic value of serum CA199, NLR and PLR levels in renal function impairment of acute cholangitis [J]. Clinical Focus, 2020, 35(6): 528-532. |
| [6] | Wang Wenjun, He Xuezhi, Gao Feng, Gao Yang, Shi Lei, Zhuang Xijing. Different DAPT effects on patency of internal thoracic artery and great saphenous vein grafts after CABG in patients with nondialysis CKD [J]. Clinical Focus, 2020, 35(1): 21-27. |
| [7] | Liu Yang, He Wei, Chinese Critical Ultrasound Study Group. Intraabdominal hypertension and renal perfusion [J]. Clinical Focus, 2019, 34(7): 584-589. |
| [8] | Chen Yunshuang, Yang Xinjun, Lin Jing, Wang Lihui, Wu Guangli. Clinical features in chronic kidney diseasemineral and bone disorder [J]. Clinical Focus, 2018, 33(10): 834-838. |
| [9] | Si Bolin, Shen Ruifang, Luo Yanping, Yu Su. Relationship of residual renal function and endothelial dysfunction in peritoneal dialysis patients [J]. Clinical Focus, 2017, 32(8): 691-694. |
| [10] | Xu Chao;Zhang Fang. Clinical features of gouty arthritis in elderly patients [J]. Clinical Focus, 2015, 30(6): 670-673. |
| [11] | GUO Chun-yan;WAND Zhong-tao;LIU Wei-ying;YU Qin. Correlation between obstructive sleep apnea-hypopnea syndrome and renal injury--a meta analysis [J]. Clinical Focus, 2014, 29(8): 841-846847. |
| [12] | BIAN Ya;JI Zi-ying;NIU Su-zhen;JIA Wan-ming;WANG Shou-shan;GU Xin-shun. Renal protective effects of different dose atorvastatin on patients with early renal damage complicated by hypertension [J]. Clinical Focus, 2014, 29(7): 733-736. |
| [13] | HUANG Wen-fu;CHEN Li-li;CHEN Hai-yun. Kidney function and immunoglobulins change in patients who underwent contrast-enhanced CT examination [J]. Clinical Focus, 2014, 29(11): 1268-1.27013e+007. |
| [14] | LIU Jin-xiang;DING Yong;WU Xiao-feng;SHAN Wei;CAO Cui-yun;REN Ying. Values of multi-parameter renal ultrasound in the differential diagnosis of acute and chronic kidney failure [J]. Clinical Focus, 2014, 29(11): 1258-1261. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||