Clinical Focus ›› 2021, Vol. 36 ›› Issue (3): 251-255.doi: 10.3969/j.issn.1004-583X.2021.03.013
Previous Articles Next Articles
Expression of PD-1 on T-lymphocytes in acute leukemia after allogeneic hematopoietic stem cell transplantation
Liu Jianning, Sun Li, Niu Zhiyun, Wen Shupeng, Wang Ying, Zhang Xuejun, Wang Fuxu( )
)
- Department of Hematology, the Second Hospital of Hebei Medical University, Hebei Key Laboratory of Hematology, Shijiazhuang 050000, China
-
Received:2020-09-23Online:2021-03-20Published:2021-03-29 -
Contact:Wang Fuxu E-mail:wfxhebmu@163.com
CLC Number:
Cite this article
Liu Jianning, Sun Li, Niu Zhiyun, Wen Shupeng, Wang Ying, Zhang Xuejun, Wang Fuxu. Expression of PD-1 on T-lymphocytes in acute leukemia after allogeneic hematopoietic stem cell transplantation[J]. Clinical Focus, 2021, 36(3): 251-255.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2021.03.013
| 预处理方案 | 例数 | 药物 | 剂量 | 时间(d) |
|---|---|---|---|---|
| mBu/Cy | 5 | Ara-c | 2 g/m2 | -9 |
| Bu | 3.2 mg/(kg·d) | -8~-6 | ||
| Cy | 1.8 g/(m2·d) | -5~-4 | ||
| MeCCNU | 250 mg/m2 | -3 | ||
| mBu/Cy+ATG | 17 | Ara-c | 4 g/(m2·d) | -10~-9 |
| Bu | 3.2 mg/(kg·d) | -8~-6 | ||
| Cy | 1.8 g/(m2·d) | -5~-4 | ||
| ATG | 2.5 mg/(kg·d) | -5~-2 | ||
| Bu/Cy+FLAG | 1 | Ara-c | 4 g/(m2·d) | -10~-9 |
| Bu | 3.2 mg/(kg·d) | -8~-6 | ||
| Cy | 40 mg/(kg·d) | -5~-4 | ||
| Flu | 30 mg/(m2·d) | -6~-2 | ||
| G-CSF | 5 μg/kg | |||
| BU/Cy+FLAG+ATG | 3 | Ara-c | 4 g/(m2·d) | -10~-9 |
| Bu | 3.2 mg/(kg·d) | -8~-6 | ||
| Cy | 40 mg/(kg·d) | -5~-4 | ||
| Flu | 30 mg/(m2·d) | -6~-2 | ||
| G- CSF | 5 μg/kg | |||
| ATG | 2.5 mg/(kg·d) | -5~-2 | ||
| TBI/Cy | 4 | TBI | 4 Gy/d | -5~-4 |
| Cy | 60 mg/(kg·d) | -3~-2 | ||
| TBI/Cy+ATG | 7(其中3例 加依托泊 苷处理) | Cy | 1.8 g/(m2·d) | -5~-4 |
| ATG | 2.5 mg/(kg·d) | -5~-2 | ||
| TBI | 4 Gy/d | -2~-1 | ||
| TBI/Cy+FLAG+ATG | 3 | Ara-c | 2 g/(m2·d) | -7~-4 |
| TBI | 4 Gy/d | -9~-8 | ||
| Cy | 40 mg/(kg·d) | -3~-2 | ||
| Flu | 30 mg/(m2·d) | -7~-4 | ||
| ATG | 2.5 mg/(kg·d) | -5~-2 | ||
| G-CSF | 5 μg/kg | -7~-4 |
| 预处理方案 | 例数 | 药物 | 剂量 | 时间(d) |
|---|---|---|---|---|
| mBu/Cy | 5 | Ara-c | 2 g/m2 | -9 |
| Bu | 3.2 mg/(kg·d) | -8~-6 | ||
| Cy | 1.8 g/(m2·d) | -5~-4 | ||
| MeCCNU | 250 mg/m2 | -3 | ||
| mBu/Cy+ATG | 17 | Ara-c | 4 g/(m2·d) | -10~-9 |
| Bu | 3.2 mg/(kg·d) | -8~-6 | ||
| Cy | 1.8 g/(m2·d) | -5~-4 | ||
| ATG | 2.5 mg/(kg·d) | -5~-2 | ||
| Bu/Cy+FLAG | 1 | Ara-c | 4 g/(m2·d) | -10~-9 |
| Bu | 3.2 mg/(kg·d) | -8~-6 | ||
| Cy | 40 mg/(kg·d) | -5~-4 | ||
| Flu | 30 mg/(m2·d) | -6~-2 | ||
| G-CSF | 5 μg/kg | |||
| BU/Cy+FLAG+ATG | 3 | Ara-c | 4 g/(m2·d) | -10~-9 |
| Bu | 3.2 mg/(kg·d) | -8~-6 | ||
| Cy | 40 mg/(kg·d) | -5~-4 | ||
| Flu | 30 mg/(m2·d) | -6~-2 | ||
| G- CSF | 5 μg/kg | |||
| ATG | 2.5 mg/(kg·d) | -5~-2 | ||
| TBI/Cy | 4 | TBI | 4 Gy/d | -5~-4 |
| Cy | 60 mg/(kg·d) | -3~-2 | ||
| TBI/Cy+ATG | 7(其中3例 加依托泊 苷处理) | Cy | 1.8 g/(m2·d) | -5~-4 |
| ATG | 2.5 mg/(kg·d) | -5~-2 | ||
| TBI | 4 Gy/d | -2~-1 | ||
| TBI/Cy+FLAG+ATG | 3 | Ara-c | 2 g/(m2·d) | -7~-4 |
| TBI | 4 Gy/d | -9~-8 | ||
| Cy | 40 mg/(kg·d) | -3~-2 | ||
| Flu | 30 mg/(m2·d) | -7~-4 | ||
| ATG | 2.5 mg/(kg·d) | -5~-2 | ||
| G-CSF | 5 μg/kg | -7~-4 |
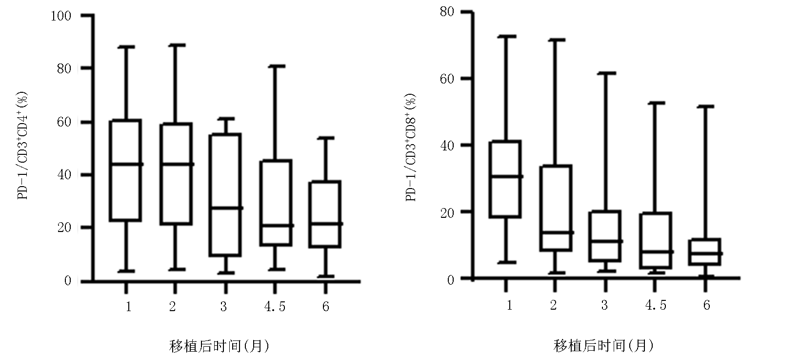
| 时间 | PD-1/CD3+CD4+ T | PD-1/CD3+CD8+ T |
|---|---|---|
| 移植后1个月 | 49.39±22.97 | 38.27±21.12 |
| 移植后2个月 | 43.75±26.75 | 28.51±20.69 |
| 移植后3个月 | 26.92±22.25 | 18.79±19.70 |
| 移植后4.5个月 | 28.59±19.83 | 17.12±18.83 |
| 移植后6个月 | 25.26±16.12 | 11.64±16.67 |
| F值 | 5.082 | 8.492 |
| P值 | 0.002 | 0.002 |
| 时间 | PD-1/CD3+CD4+ T | PD-1/CD3+CD8+ T |
|---|---|---|
| 移植后1个月 | 49.39±22.97 | 38.27±21.12 |
| 移植后2个月 | 43.75±26.75 | 28.51±20.69 |
| 移植后3个月 | 26.92±22.25 | 18.79±19.70 |
| 移植后4.5个月 | 28.59±19.83 | 17.12±18.83 |
| 移植后6个月 | 25.26±16.12 | 11.64±16.67 |
| F值 | 5.082 | 8.492 |
| P值 | 0.002 | 0.002 |
| [1] |
Frassoni F, Barrett AJ, Granena A, et al. Relapse after allogeneic bone marrow transplantation for acute leukaemia: a survey by the E.B. M.T. of 117 cases[J]. Br J Haematol, 1988,70(3):317-320.
doi: 10.1111/j.1365-2141.1988.tb02488.x URL |
| [2] |
Mielcarek M, Storer BE, Flowers ME, et al. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation[J]. Biol Blood Marrow Transplant, 2007,13(10):1160-1168.
doi: 10.1016/j.bbmt.2007.06.007 URL |
| [3] |
Fathi AT, Chen YB. Treatment of relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation[J]. Curr Hematol Malig Rep, 2014,9(2):186-192.
doi: 10.1007/s11899-014-0209-2 pmid: 24643311 |
| [4] | 张之南, 沈悌. 《血液病诊断及疗效标准》[M].3版. 北京: 科学出版社, 2007. |
| [5] | 中华医学会血液学分会. 成人急性髓系白血病(非急性早幼粒细胞白血病)中国诊疗指南(2017年版)[J]. 中华血液学杂志, 2017,38(3):177-182. |
| [6] | 中国抗癌协会血液肿瘤专业委员会. 中国成人急性淋巴细胞白血病诊断与治疗指南(2016年版)[J]. 中华血液学杂志, 2016,37(10):837-845. |
| [7] | 宁红梅, 冯凯, 陈虎, 等. 粘附分子与移植物抗宿主病[J]. 国外医学.输血及血液学分册, 2004,27(2):102-106. |
| [8] | Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy[J]. Net Rev Immunol, 2012,12(6):443-458. |
| [9] |
Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD[J]. Blood, 2009,114(3):702-708.
doi: 10.1182/blood-2009-03-208983 pmid: 19470693 |
| [10] | 中华医学会血液学分会干细胞应用学组. 中国异基因造血干细胞移植治疗血液系统疾病专家共识(I)—适应症、预处理方案及供者选择(2014年版)[J]. 中华血液学杂志, 2014,35(8):775-780. |
| [11] | Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading[J]. Bone Marrow Transplant, 1995,15(6):825-828. |
| [12] |
Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I.Diagnosis and staging working group report[J]. Biol Blood Marrow Transplant, 2005,11(12):945-956.
doi: 10.1016/j.bbmt.2005.09.004 URL |
| [13] | 中华医学会血液学分会干细胞应用学组. 中国异基因造血干细胞移植治疗血液系统疾病专家共识(III)—急性移植物抗宿主病(2020年版)[J]. 中华血液学杂志, 2020,41(7):529-536. |
| [14] |
Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity[J]. Autoimmun Rev, 2013,12(11):1091-1100.
doi: 10.1016/j.autrev.2013.05.003 pmid: 23792703 |
| [15] | 孙建军, 冯辉. CD8+T细胞耗竭及靶向CD8+T细胞免疫治疗的研究进展[J]. 免疫学杂志, 2016,32(9):816-820. |
| [16] |
Simonetta F, Pradier A, Bosshard C, et al. Dynamics of expression of Programmed Cell Death Protein-1 (PD-1) on T cells after allogeneic hematopoietic stem cell transplantation[J]. Front Immunol, 2019,10:1034.
doi: 10.3389/fimmu.2019.01034 pmid: 31156625 |
| [17] |
Kong Y, Zhang J, Claxton DF, et al. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation[J]. Blood Cancer J, 2015,5(7):e330.
doi: 10.1038/bcj.2015.58 URL |
| [18] |
Noviello M, Manfredi F, Ruggiero E, et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT[J]. Nat Commun, 2019,10(1):1065.
doi: 10.1038/s41467-019-08871-1 URL |
| [19] |
Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies[J]. Clin Cancer Res, 2008,14(10):3044-3051.
doi: 10.1158/1078-0432.CCR-07-4079 URL |
| [20] |
Ravandi F, Assi R, Daver N, et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study[J]. Lancet Haematol, 2019,6(9):e480-e488.
doi: 10.1016/S2352-3026(19)30114-0 pmid: WOS:000483400400011 |
| [21] |
Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD[J]. Blood, 2017,130(2):221-228.
doi: 10.1182/blood-2017-01-761346 pmid: 28468799 |
| [22] |
Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation[J]. Blood, 2009,113(7):1581-1588.
doi: 10.1182/blood-2008-07-168468 pmid: 18974373 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||