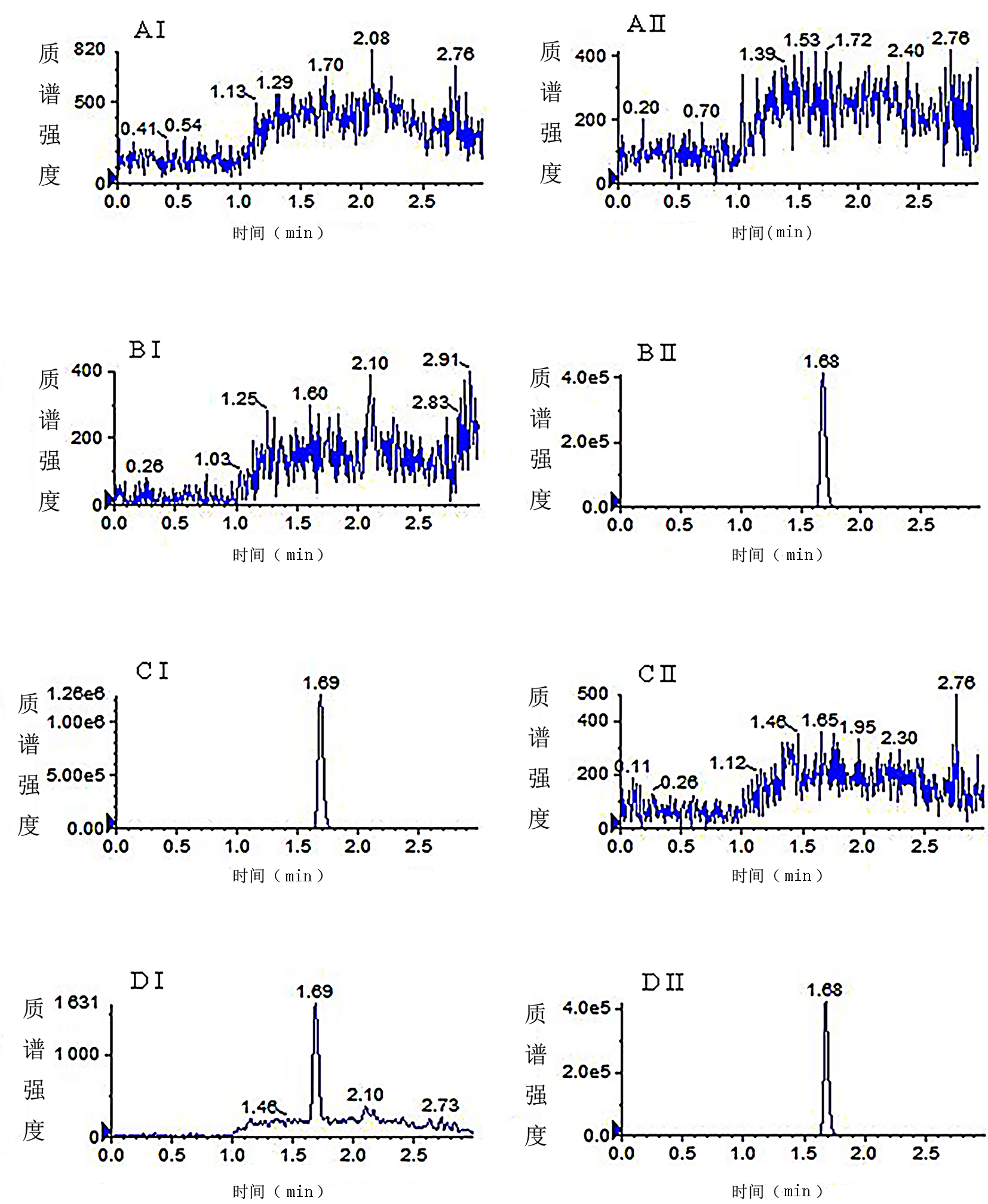
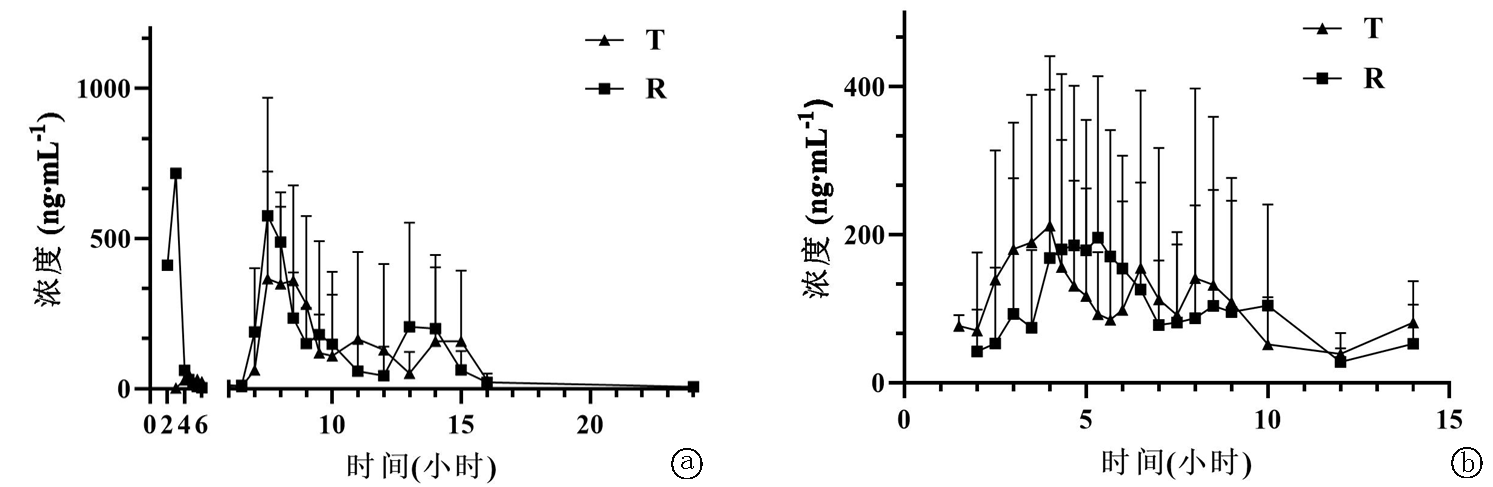
| [1] |
Sai Y, Kusaka A, Imanishi K, et al. A randomized, quadruple crossover single-blind study on immediate action of chewed and unchewed low-dose acetylsalicylic acid tablets in healthy volunteers[J]. J Pharm Sci, 2011, 100(9):3884-3891.
|
| [2] |
张嘉祺, 王茜婷, 刘梅林. 阿司匹林在心血管疾病二级预防中的研究进展[J]. 中国循环杂志, 2023, 38(8):887-890.
|
| [3] |
王静, 李丽, 陈思, 等. 阿司匹林肠溶片的生物等效性试验设计要点及审评考虑[J]. 中国新药杂志, 2022, 31(23):2361-2364.
|
| [4] |
Zhou XJ, Tian J, Zhu MZ, et al. A systematic review and meta-analysis of published randomized controlled trials of combination of clopidogrel and aspirin in transient ischemic attack or minor stroke[J]. Exp Ther Med, 2017, 14(1):324-332.
doi: 10.3892/etm.2017.4459
pmid: 28672933
|
| [5] |
徐静, 白洋. 中国高血压临床实践指南[J]. 中华心血管病杂志, 2022, 50(11):1050-1095.
|
| [6] |
郭平平, 陈晓霞, 王晓蓉, 等. 阿司匹林和氯吡格雷抗血小板抵抗机制及临床治疗研究进展[J]. 中国临床神经科学, 2019, 27(3):321-328.
|
| [7] |
国家药品监督管理局. 以药动学参数为终点评价指标的化学药物仿制药人体生物等效性研究技术指导原则[S]. 2016:13-14.
|
| [8] |
国家药品监督管理局. 高变异药物生物等效性研究技术指导原则[S]. 2018:3.
|
| [9] |
刘曼, 张丹, 王晓琳, 等. 高变异药物及其参比制剂校正的平均生物等效性试验的探讨[J]. 中国新药杂志, 2014, 23(2):257-260.
|
| [10] |
刘月, 蔡晶晶, 于宣宣. 高变异药物生物等效性分析方法的比较[J]. 定量药理学, 2019, 7(24):792-798.
|
| [11] |
杨辉, 周远大, 李娟, 等. 标准化全流程管理在高变异药物生物等效性评价中的应用研究[J]. 中国药业, 2020, 29(18):1-4.
|
| [12] |
国家药品监督管理局. 阿司匹林肠溶片生物等效性研究技术指导原则[S]. 2022:9.
|
| [13] |
Van Den Abeele J, Rubbens J, Brouwers J, et al. The dynamic gastric environment and its impact on drug and formulation behaviour[J]. Eur J Pharm Sci, 2017(96):207-231.
|
| [14] |
Raju NC, Eikelboom JW. The aspirin controversy in primary prevention[J]. Curr Opin Cardiol, 2012, 27(5):499-507.
doi: 10.1097/HCO.0b013e328356ae95
pmid: 22874127
|
| [15] |
Wang L, Di Y, Guo T, et al. Implementation of a reference-scaled average bioequivalence approach for highly variable acetylsalicylic acid in fixed-dose combination with clopidogrel versus enteric aspirin in Chinese subjects under fasting conditions: A phase 1, open-label, randomized, crossover study[J]. Adv Ther, 2020, 37(6):2696-2709.
doi: 10.1007/s12325-020-01369-z
pmid: 32418143
|
| [16] |
Li Y, Ming JE, Kong F, et al. Bioequivalence study comparing fixed-dose combination of clopidogrel and aspirin with coadministration of individual formulations in chinese subjects under fed conditions: A phase Ⅰ, open-label, randomized, crossover study[J]. Adv Ther, 2020, 37(11):4660-4674.
|
| [17] |
Charman WN, Porter CJ, Mithani S, et al. Physicochemical and physiological mechanisms for the effects of food on drug absorption: The role of lipids and PH[J]. J Pharm Sci-us, 1997, 86(3):269-282.
pmid: 9050793
|
| [18] |
Rimon G, Sidhu RS, Lauver DA, et al. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1[J]. Proc Natl Acad Sci USA, 2010, 107(1):28-33.
doi: 10.1073/pnas.0909765106
pmid: 19955429
|
| [19] |
高志峰, 赵媛媛, 周杰. 阿司匹林肠溶片生物等效性试验[J]. 药物分析杂志, 2016, 2:217-225.
|
| [20] |
石蕊, 郝颖翠, 李艳华, 等. 硫酸氢氯吡格雷阿司匹林片在健康志愿者中空腹及餐后生物等效性评价[J]. 中国医院药学杂志, 2023, 43(21):2379-2384.
doi: 10.13286/j.1001-5213.2023.21.04
|
| [21] |
黄京山, 李伟, 邵伟. 阿司匹林肠溶片的制备及其稳定性考察[J]. 山东化工, 2022, 51(24):19-21.
|
| [22] |
侯飞, 罗四海, 方夏琴, 等. 阿司匹林肠溶片工艺与体外质量一致性研究[J]. 中国医药导报, 2019, 16(3):101-104.
|
| [23] |
葛庆华, 丁存刚, 周臻, 等. 阿司匹林肠溶胶囊与肠溶片在健康人体内药动学比较[J]. 世界临床药物, 2014, 10:605-610.
|
| [24] |
Angiolillo DJ, Bhatt DL, Lanza F, et al. Pharmacokinetic/pharmacodynamic assessment of a novel, pharmaceutical lipid-aspirin complex: Results of a randomized, crossover, bioequivalence study[J]. J Thromb Thrombolysis, 2019, 48(4):554-562.
|
| [25] |
王瑜, 佟媛旭, 张倩, 等. 高变异药物生物等效性研究的样本全生命周期模式质量管理的探讨[J]. 中国临床药理学杂志, 2020, 36(17):2717-2720.
|
 )
)