Clinical Focus ›› 2023, Vol. 38 ›› Issue (1): 37-41.doi: 10.3969/j.issn.1004-583X.2023.01.003
Previous Articles Next Articles
Correlation between ratio of salivary uric acid to blood uric acid and diabetic peripheral neuropathy
Ju Yan1, Guo Peng3, Wu Botao1, Liu Xinyu2( )
)
- 1. Graduate School,Jinzhou Medical University,Jinzhou 121000,China
2. Department of Endocrinology, the First Affiliated Hospital of Jinzhou Medical University,Jinzhou 121000,China
3. Department of Cardiology,Liaoyang City Central Hospital,Liaoyang 111000,China
-
Received:2022-07-24Online:2023-01-20Published:2023-03-03 -
Contact:Liu Xinyu E-mail:xxy-005@163.com
CLC Number:
Cite this article
Ju Yan, Guo Peng, Wu Botao, Liu Xinyu. Correlation between ratio of salivary uric acid to blood uric acid and diabetic peripheral neuropathy[J]. Clinical Focus, 2023, 38(1): 37-41.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2023.01.003
| 组别 | T2DM ( | DPN ( | 统计值 | |
|---|---|---|---|---|
| 年龄(岁) | 58.61±5.78 | 57.8±6.01 | 0.77 | 0.445 |
| 性别(男)[例(%)] | 34(55.7) | 40(38.9) | 3.16 | 0.083 |
| BMI(kg/m2)[例(%)] | ||||
| ≤24 | 25(41.0) | 30(29.1) | ||
| >24~28 | 31(50.8) | 54(52.4) | 4.38 | 0.114 |
| >28 | 5(8.2) | 19(18.5) | ||
| DM病程(年)[例(%)] | ||||
| ≤5 | 20(32.8) | 34(33.0) | ||
| 5~10 | 30(49.2) | 46(44.7) | 0.51 | 0.781 |
| >10 | 11(18.0) | 23(22.3) | ||
| 高血压病史[例(%)] | 22(36.1) | 33(32.0) | 0.28 | 0.601 |
| FPG(mmol/L) | 9.83±3.60 | 10.14±3.61 | -0.54 | 0.592 |
| HbA1c(%) | 8.55±2.64 | 9.50±2.02 | -2.59 | 0.010 |
| TC(mmol/L) | 4.76±1.17 | 4.82±1.35 | -0.29 | 0.774 |
| TG(mmol/L) | 1.98±1.80 | 2.63±1.91 | -2.18 | 0.031 |
| HDL-C(mmol/L) | 1.22±0.53 | 1.14±0.50 | 0.89 | 0.385 |
| LDL-C(mmol/L) | 2.42±0.63 | 2.52±0.81 | -0.79 | 0.433 |
| BUN(mmol/L) | 5.82±1.84 | 6.37±2.17 | -1.67 | 0.104 |
| Scr(μmol/L) | 58.46±19.10 | 63.71±18.73 | -1.72 | 0.090 |
| BUA(μmol/L) | 358.16±143.17 | 324.65±173.53 | 1.27 | 0.215 |
| SUA(μmol/L) | 328.07±76.16 | 393.76±104.83 | -4.27 | 0.001 |
| SUA/BUA | 1.02±0.41 | 1.43±0.55 | -5.30 | 0.001 |
Tab.1 Comparison of general data
| 组别 | T2DM ( | DPN ( | 统计值 | |
|---|---|---|---|---|
| 年龄(岁) | 58.61±5.78 | 57.8±6.01 | 0.77 | 0.445 |
| 性别(男)[例(%)] | 34(55.7) | 40(38.9) | 3.16 | 0.083 |
| BMI(kg/m2)[例(%)] | ||||
| ≤24 | 25(41.0) | 30(29.1) | ||
| >24~28 | 31(50.8) | 54(52.4) | 4.38 | 0.114 |
| >28 | 5(8.2) | 19(18.5) | ||
| DM病程(年)[例(%)] | ||||
| ≤5 | 20(32.8) | 34(33.0) | ||
| 5~10 | 30(49.2) | 46(44.7) | 0.51 | 0.781 |
| >10 | 11(18.0) | 23(22.3) | ||
| 高血压病史[例(%)] | 22(36.1) | 33(32.0) | 0.28 | 0.601 |
| FPG(mmol/L) | 9.83±3.60 | 10.14±3.61 | -0.54 | 0.592 |
| HbA1c(%) | 8.55±2.64 | 9.50±2.02 | -2.59 | 0.010 |
| TC(mmol/L) | 4.76±1.17 | 4.82±1.35 | -0.29 | 0.774 |
| TG(mmol/L) | 1.98±1.80 | 2.63±1.91 | -2.18 | 0.031 |
| HDL-C(mmol/L) | 1.22±0.53 | 1.14±0.50 | 0.89 | 0.385 |
| LDL-C(mmol/L) | 2.42±0.63 | 2.52±0.81 | -0.79 | 0.433 |
| BUN(mmol/L) | 5.82±1.84 | 6.37±2.17 | -1.67 | 0.104 |
| Scr(μmol/L) | 58.46±19.10 | 63.71±18.73 | -1.72 | 0.090 |
| BUA(μmol/L) | 358.16±143.17 | 324.65±173.53 | 1.27 | 0.215 |
| SUA(μmol/L) | 328.07±76.16 | 393.76±104.83 | -4.27 | 0.001 |
| SUA/BUA | 1.02±0.41 | 1.43±0.55 | -5.30 | 0.001 |
| 自变量 | Wald χ2值 | 95% | ||||
|---|---|---|---|---|---|---|
| HbA1c | 0.18 | 0.09 | 4.21 | 0.040 | 1.19 | 1.008~1.410 |
| TG | 0.17 | 0.11 | 2.35 | 0.131 | 1.19 | 0.953~1.484 |
| SUA | 0.01 | 0.02 | 10.95 | 0.001 | 1.01 | 1.003~1.013 |
| SUA/BUA | 1.90 | 0.47 | 16.71 | 0.001 | 6.67 | 2.691~16.674 |
Tab.2 Logistic regression analysis for DPN-related risk factors
| 自变量 | Wald χ2值 | 95% | ||||
|---|---|---|---|---|---|---|
| HbA1c | 0.18 | 0.09 | 4.21 | 0.040 | 1.19 | 1.008~1.410 |
| TG | 0.17 | 0.11 | 2.35 | 0.131 | 1.19 | 0.953~1.484 |
| SUA | 0.01 | 0.02 | 10.95 | 0.001 | 1.01 | 1.003~1.013 |
| SUA/BUA | 1.90 | 0.47 | 16.71 | 0.001 | 6.67 | 2.691~16.674 |
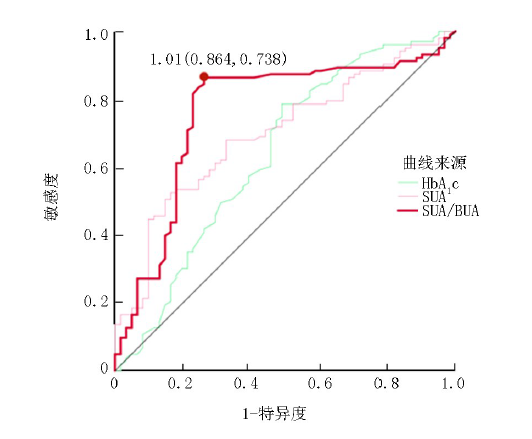
| 变量 | AUC | SE | 敏感度 | 特异度 | 切点 | 95% | |
|---|---|---|---|---|---|---|---|
| HbA1c | 0.637 | 0.047 | 0.508 | 0.786 | 8.15 | 0.003 | 0.545~0.729 |
| SUA | 0.698 | 0.041 | 0.524 | 0.836 | 388.43 | 0.001 | 0.617~0.779 |
| SUA/BUA | 0.762 | 0.042 | 0.864 | 0.738 | 1.01 | 0.001 | 0.680~0.843 |
Tab.3 Comparison of the diagnostic efficacy of HbA1c, SUA, SUA/BUA for DPN
| 变量 | AUC | SE | 敏感度 | 特异度 | 切点 | 95% | |
|---|---|---|---|---|---|---|---|
| HbA1c | 0.637 | 0.047 | 0.508 | 0.786 | 8.15 | 0.003 | 0.545~0.729 |
| SUA | 0.698 | 0.041 | 0.524 | 0.836 | 388.43 | 0.001 | 0.617~0.779 |
| SUA/BUA | 0.762 | 0.042 | 0.864 | 0.738 | 1.01 | 0.001 | 0.680~0.843 |
| [1] |
Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes[J]. Curr Diab Rep, 2019, 19(10):86.
doi: 10.1007/s11892-019-1212-8 URL |
| [2] |
Liu X, Xu Y, An M, et al. The risk factors for diabetic peripheral neuropathy: A meta-analysis[J]. PLoS One, 2019, 14(2):e0212574.
doi: 10.1371/journal.pone.0212574 URL |
| [3] |
Armstrong DG, Boulton A, Bus SA. Diabetic foot ulcers and their recurrence[J]. N Engl J Med, 2017, 376(24): 2367-2375.
doi: 10.1056/NEJMra1615439 URL |
| [4] |
Zhang P, Lu J, Jing Y, et al. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis[J]. Ann Med, 2017, 49(2): 106-116.
doi: 10.1080/07853890.2016.1231932 URL |
| [5] |
Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention[J]. Lancet Diabetes Endocrinol, 2019, 7(12): 938-948.
doi: 10.1016/S2213-8587(19)30081-6 URL |
| [6] |
Yu S, Chen Y, Hou X, et al. Serum uric acid levels and diabetic peripheral neuropathy in type 2 diabetes: A systematic review and meta-analysis[J]. Mol Neurobiol, 2016, 53(2):1045-1051.
doi: 10.1007/s12035-014-9075-0 pmid: 25579387 |
| [7] |
Carmichael J, Fadavi H, Ishibashi F, et al. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy[J]. Front Endocrinol (Lausanne), 2021, 12:671257.
doi: 10.3389/fendo.2021.671257 URL |
| [8] |
Corey-Bloom J, Haque A, Aboufadel S, et al. Uric acid as a potential peripheral biomarker for disease features in Huntington's patients[J]. Front Neurosci, 2020, 14:73.
doi: 10.3389/fnins.2020.00073 URL |
| [9] |
Vernerová A, Kujovská Krčmová L, Melichar B, et al. Non-invasive determination of uric acid in human saliva in the diagnosis of serious disorders[J]. Clin Chem Lab Med, 2021, 59(5):797-812.
doi: 10.1515/cclm-2020-1533 pmid: 33554551 |
| [10] |
Araujo DS, Scudine K, Pedroni-Pereira A, et al. Salivary uric acid is a predictive marker of body fat percentage in adolescents[J]. Nutr Res, 2020, 74:62-70.
doi: S0271-5317(19)30730-4 pmid: 31954275 |
| [11] |
Kaewput W, Thongprayoon C, Rangsin R, et al. The association between serum uric acid and peripheral neuropathy in patients with type 2 diabetes mellitus: A multicenter nationwide crosssectional study[J]. Korean J Fam Med, 2020, 41(3):189-194.
doi: 10.4082/kjfm.18.0205 pmid: 32456387 |
| [12] | Maciejczyk M, Pawlukianiec C, Żendzian-Piotrowska M, et al. Salivary redox biomarkers in insulin resistance: Preclinical studies in an animal model[J]. Oxid Med Cell Longev, 2021, 2021: 3734252. |
| [13] | 中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志, 2021, 13(4): 315-409. |
| [14] | 中华医学会糖尿病学分会神经并发症学组. 糖尿病神经病变诊治专家共识(2021年版)[J]. 中华内分泌代谢杂志, 2021, 37(6): 499-515. |
| [15] |
Klimiuk A, Zalewska A, Sawicki R, et al. Salivary oxidative stress increases with the progression of chronic heart failure[J]. J Clin Med, 2020, 9(3):769.
doi: 10.3390/jcm9030769 URL |
| [16] |
Maciejczyk M, Taranta-Janusz K, Wasilewska A, et al. A case-control study of salivary redox homeostasis in hypertensive children. Can salivary uric acid be a marker of hypertension?[J]. J Clin Med, 2020, 9(3):837.
doi: 10.3390/jcm9030837 URL |
| [17] | Maciejczyk M, Bielas M, Zalewska A, et al. Salivary biomarkers of oxidative stress and inflammation in stroke patients: From basic research to clinical practice[J]. Oxid Med Cell Longev, 2021, 2021: 5545330. |
| [18] |
Feldman EL, Nave KA, Jensen TS, et al. New horizons in diabetic neuropathy: Mechanisms, bioenergetics, and pain[J]. Neuron, 2017, 93(6):1296-1313.
doi: S0896-6273(17)30089-2 pmid: 28334605 |
| [19] |
Baum P, Toyka KV, Blüher M, et al. Inflammatory mechanisms in the pathophysiology of diabetic peripheral neuropathy (DN)-new aspects[J]. Int J Mol Sci, 2021, 22(19):10835.
doi: 10.3390/ijms221910835 URL |
| [20] |
Lupachyk S, Watcho P, Hasanova N, et al. Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: Role for oxidative-nitrosative stress[J]. Free Radic Biol Med, 2012, 52(8):1255-1263.
doi: 10.1016/j.freeradbiomed.2012.01.029 URL |
| [21] |
Xu T, Weng Z, Pei C, et al. The relationship between neutrophil-to-lymphocyte ratio and diabetic peripheral neuropathy in type 2 diabetes mellitus[J]. Medicine (Baltimore), 2017, 96(45):e8289.
doi: 10.1097/MD.0000000000008289 URL |
| [22] |
Soukup M, Biesiada I, Henderson A, et al. Salivary uric acid as a noninvasive biomarker of metabolic syndrome[J]. Diabetol Metab Syndr, 2012, 4(1):14.
doi: 10.1186/1758-5996-4-14 pmid: 22515434 |
| [23] | Maciejczyk M, Gerreth P, Zalewska A, et al. Salivary gland dysfunction in stroke patients is associated with increased protein glycoxidation and nitrosative stress[J]. Oxid Med Cell Longev, 2020, 2020:6619439. |
| [24] |
Younus H, Ahmad S, Alam MF. Correlation between the activity of aldehyde dehydrogenase and oxidative stress markers in the saliva of diabetic patients[J]. Protein Pept Lett, 2020, 27(1):67-73.
doi: 10.2174/0929866526666191002115121 URL |
| [25] |
Stavniichuk R, Shevalye H, Lupachyk S, et al. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy[J]. Diabetes Metab Res Rev, 2014, 30(8):669-678.
doi: 10.1002/dmrr.2549 URL |
| [26] | Sifuentes-Franco S, Pacheco-Moisés FP, Rodríguez-Carrizalez AD, et al. The role of oxidative stress, mitochondrial function, and autophagy in diabetic polyneuropathy[J]. J Diabetes Res, 2017, 2017: 1673081. |
| [27] |
Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics[J]. Diab Vasc Dis Res, 2011, 8(1):22-28.
doi: 10.1177/1479164110390243 URL |
| [28] | Żukowski P, Maciejczyk M, Waszkiel D. Sources of free radicals and oxidative stress in the oral cavity[J]. Arch Oral Biol,2018, 92: 8-17. |
| [29] |
Maciejczyk M, Zalewska A, Gerreth AK. Salivary redox biomarkers in selected neurodegenerative diseases[J]. J Clin Med, 2020, 9(2):497.
doi: 10.3390/jcm9020497 URL |
| [30] |
Zalewska A, Klimiuk A, Zięba S, et al. Salivary gland dysfunction and salivary redox imbalance in patients with Alzheimer's disease[J]. Sci Rep, 2021, 11(1):23904.
doi: 10.1038/s41598-021-03456-9 pmid: 34903846 |
| [31] |
Fathi S, Borzouei S, Goodarzi MT, et al. Evaluation of salivary antioxidants and oxidative stress markers in type 2 diabetes mellitus: A retrospective cohort study[J]. Endocr Metab Immune Disord Drug Targets, 2020, 20(4): 584-590.
doi: 10.2174/1871530319666191016103222 pmid: 31622212 |
| [32] |
Casadei G, Filippini M, Brognara L. Glycated hemoglobin (HbA1c) as a biomarker for diabetic foot peripheral neuropathy[J]. Diseases, 2021, 9(1):16.
doi: 10.3390/diseases9010016 URL |
| [33] |
Su JB, Zhao LH, Zhang XL, et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients[J]. Cardiovasc Diabetol, 2018, 17(1):47.
doi: 10.1186/s12933-018-0693-0 URL |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 50
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 217
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||