Clinical Focus ›› 2022, Vol. 37 ›› Issue (3): 197-203.doi: 10.3969/j.issn.1004-583X.2022.03.001
The efficacy and safety of different blood pressure target on secondary stroke prevention: A systematic review and meta-analysis
Wang Xiaoqing1, Guo Yijia2, Tang Yifang2, Tang Qin2, Yang Jie2( )
)
- 1. Public Health Clinical Center of Chengdu, Chengdu 610500, China
2. Chengdu Medical College, Chengdu 610500, China
-
Received:2020-11-03Online:2022-03-20Published:2022-04-02 -
Contact:Yang Jie E-mail:892878026@qq.com
CLC Number:
Cite this article
Wang Xiaoqing, Guo Yijia, Tang Yifang, Tang Qin, Yang Jie. The efficacy and safety of different blood pressure target on secondary stroke prevention: A systematic review and meta-analysis[J]. Clinical Focus, 2022, 37(3): 197-203.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2022.03.001
| 纳入研究 (年) | 研究 类型 | 国家* | 样本量 (T/C, 例) | 平均年龄 (T/C, 岁) | 男性 (T/C, 例) | 干预措施 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | ||||||||||||||||
| RESPCT 2019 | RCT | 日本 | 633/630 | 67.2/67.3 | 449/428 | 目标收缩压<120 mmHg | 目标收缩压<140 mmHg | ||||||||||
| PODCAST 2017 | RCT | 英国 | 41/42 | 73.0/75.1 | 33/31 | 目标收缩压<125 mmHg | 目标收缩压<140 mmHg | ||||||||||
| PAST-BP 2016 | RCT | 英国 | 266/263 | 71.9/71.1 | 157/156 | 目标收缩压<125 mmHg | 目标收缩压<140 mmHg | ||||||||||
| SPS3 2013 | RCT | 北美 | 1 519/1 501 | 63/63 | 990/912 | 目标收缩压<130 mmHg | 目标收缩压<149 mmHg | ||||||||||
| PRoFESS 2008 | RCT | 加拿大 | 10 146/10 186 | 66.1/66.2 | 6 527/6 495 | 替米沙坦 | 安慰剂 | ||||||||||
| PROGRESS 2001 | RCT | 澳大利亚 | 3 051/3 054 | 64/64 | 2 128/2 125 | 培哚普利和(或)吲达帕胺 | 安慰剂 | ||||||||||
| HOPE 2000 | RCT | 加拿大 | 500/513 | 66/66 | 362/380 | 雷米普利 | 安慰剂 | ||||||||||
| PATS 1995 | RCT | 中国 | 2 840/2 825 | 60.1/60.4 | 2 040/2 033 | 吲达帕胺 | 安慰剂 | ||||||||||
| Dutch TIA 1993 | RCT | 荷兰 | 732/741 | 54/55 | 66/62 | 阿替洛尔 | 安慰剂 | ||||||||||
| HSCSG 1974 | RCT | 美国 | 233/219 | 59.0/59.1 | 139/126 | 地舍平和甲氯噻嗪 | 安慰剂 | ||||||||||
| 纳入研究 (年) | 随访时间 (年) | 基线收缩压 (T/C, mmHg) | 基线舒张压 (T/C, mmHg) | 目标收缩压 (T/C, mmHg) | 终末收缩压 (T/C, mmHg) | 结局指标 | |||||||||||
| RESPCT 2019 | 3.5 | 145.1/145.7 | 83.0/83.7 | <120/<120~140 | 126.7/133.2 | ①②③④⑤ | |||||||||||
| PODCAST 2017 | 2 | 145.9/148.3 | 82.5/81.7 | <125/<140 | NR | ①③④⑤ | |||||||||||
| PAST-BP 2016 | 1 | 143.5/142.2 | 78.8/80.7 | <125/<140 | 127.4/129.4 | ①③④⑤ | |||||||||||
| SPS3 2013 | 3.7 | 144/142 | 79/78 | <130/<130~149 | 127/138 | ①③④⑤ | |||||||||||
| PRoFESS 2008 | 2.5 | 144.1/144.2 | 84/84 | NR | 137/141 | ①③④⑤ | |||||||||||
| PROGRESS 2001 | 3.9 | 147/147 | 86/86 | NR | 135/144 | ①②③④⑤ | |||||||||||
| HOPE 2000 | 5 | 139/139 | 79/79 | NR | 136/139 | ① | |||||||||||
| PATS 1995 | 2 | 154.0/153.6 | 93.0/92.6 | NR | 141.4/146.9 | ①②③④⑤ | |||||||||||
| Dutch TIA 1993 | 2.6 | 158/157 | 91/91 | NR | 149/159 | ①②③④ | |||||||||||
| HSCSG 1974 | 3 | 167/167 | 100/100 | NR | 142/167 | ①③⑤⑥ | |||||||||||
| 纳入研究 (年) | 研究 类型 | 国家* | 样本量 (T/C, 例) | 平均年龄 (T/C, 岁) | 男性 (T/C, 例) | 干预措施 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | ||||||||||||||||
| RESPCT 2019 | RCT | 日本 | 633/630 | 67.2/67.3 | 449/428 | 目标收缩压<120 mmHg | 目标收缩压<140 mmHg | ||||||||||
| PODCAST 2017 | RCT | 英国 | 41/42 | 73.0/75.1 | 33/31 | 目标收缩压<125 mmHg | 目标收缩压<140 mmHg | ||||||||||
| PAST-BP 2016 | RCT | 英国 | 266/263 | 71.9/71.1 | 157/156 | 目标收缩压<125 mmHg | 目标收缩压<140 mmHg | ||||||||||
| SPS3 2013 | RCT | 北美 | 1 519/1 501 | 63/63 | 990/912 | 目标收缩压<130 mmHg | 目标收缩压<149 mmHg | ||||||||||
| PRoFESS 2008 | RCT | 加拿大 | 10 146/10 186 | 66.1/66.2 | 6 527/6 495 | 替米沙坦 | 安慰剂 | ||||||||||
| PROGRESS 2001 | RCT | 澳大利亚 | 3 051/3 054 | 64/64 | 2 128/2 125 | 培哚普利和(或)吲达帕胺 | 安慰剂 | ||||||||||
| HOPE 2000 | RCT | 加拿大 | 500/513 | 66/66 | 362/380 | 雷米普利 | 安慰剂 | ||||||||||
| PATS 1995 | RCT | 中国 | 2 840/2 825 | 60.1/60.4 | 2 040/2 033 | 吲达帕胺 | 安慰剂 | ||||||||||
| Dutch TIA 1993 | RCT | 荷兰 | 732/741 | 54/55 | 66/62 | 阿替洛尔 | 安慰剂 | ||||||||||
| HSCSG 1974 | RCT | 美国 | 233/219 | 59.0/59.1 | 139/126 | 地舍平和甲氯噻嗪 | 安慰剂 | ||||||||||
| 纳入研究 (年) | 随访时间 (年) | 基线收缩压 (T/C, mmHg) | 基线舒张压 (T/C, mmHg) | 目标收缩压 (T/C, mmHg) | 终末收缩压 (T/C, mmHg) | 结局指标 | |||||||||||
| RESPCT 2019 | 3.5 | 145.1/145.7 | 83.0/83.7 | <120/<120~140 | 126.7/133.2 | ①②③④⑤ | |||||||||||
| PODCAST 2017 | 2 | 145.9/148.3 | 82.5/81.7 | <125/<140 | NR | ①③④⑤ | |||||||||||
| PAST-BP 2016 | 1 | 143.5/142.2 | 78.8/80.7 | <125/<140 | 127.4/129.4 | ①③④⑤ | |||||||||||
| SPS3 2013 | 3.7 | 144/142 | 79/78 | <130/<130~149 | 127/138 | ①③④⑤ | |||||||||||
| PRoFESS 2008 | 2.5 | 144.1/144.2 | 84/84 | NR | 137/141 | ①③④⑤ | |||||||||||
| PROGRESS 2001 | 3.9 | 147/147 | 86/86 | NR | 135/144 | ①②③④⑤ | |||||||||||
| HOPE 2000 | 5 | 139/139 | 79/79 | NR | 136/139 | ① | |||||||||||
| PATS 1995 | 2 | 154.0/153.6 | 93.0/92.6 | NR | 141.4/146.9 | ①②③④⑤ | |||||||||||
| Dutch TIA 1993 | 2.6 | 158/157 | 91/91 | NR | 149/159 | ①②③④ | |||||||||||
| HSCSG 1974 | 3 | 167/167 | 100/100 | NR | 142/167 | ①③⑤⑥ | |||||||||||
| 结局指标 | 纳入研究 数量 | RR(95%CI) | P值 | I2 (%) | P for Cochran Q |
|---|---|---|---|---|---|
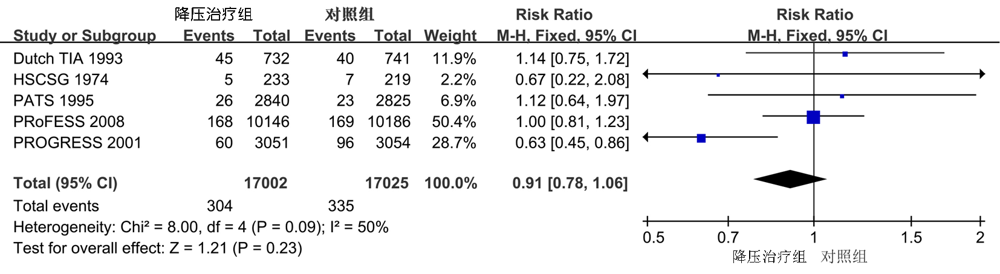
| 主要血管性事件 | 4 | 0.82(0.69,0.97) | 0.02 | 5 | 0.37 |
| 卒中 | 4 | 0.78(0.64,0.95) | 0.01 | 0 | 0.58 |
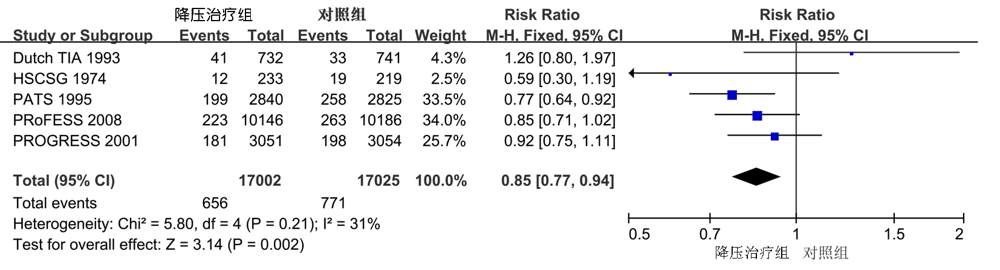
| 心肌梗死 | 4 | 0.92(0.61,1.39) | 0.7 | 0 | 0.9 |
| 血管性死亡 | 2 | 0.87(0.56,1.34) | 0.53 | 0 | 0.55 |
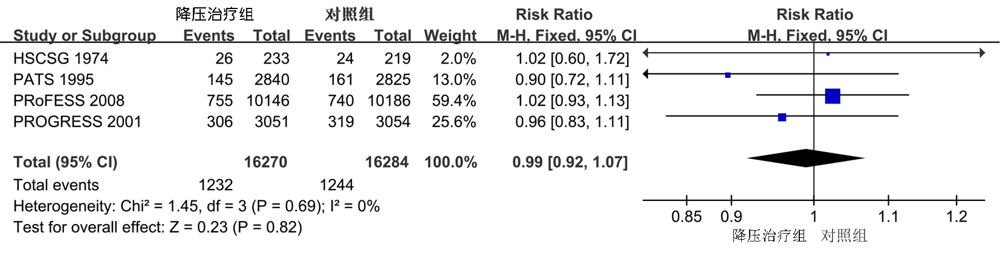
| 全因死亡 | 4 | 0.92(0.74,1.16) | 0.49 | 0 | 0.8 |
| 结局指标 | 纳入研究 数量 | RR(95%CI) | P值 | I2 (%) | P for Cochran Q |
|---|---|---|---|---|---|
| 主要血管性事件 | 4 | 0.82(0.69,0.97) | 0.02 | 5 | 0.37 |
| 卒中 | 4 | 0.78(0.64,0.95) | 0.01 | 0 | 0.58 |
| 心肌梗死 | 4 | 0.92(0.61,1.39) | 0.7 | 0 | 0.9 |
| 血管性死亡 | 2 | 0.87(0.56,1.34) | 0.53 | 0 | 0.55 |
| 全因死亡 | 4 | 0.92(0.74,1.16) | 0.49 | 0 | 0.8 |
| [1] |
O'Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study[J]. Lancet, 2016, 388(10046):761-775.
doi: 10.1016/S0140-6736(16)30506-2 pmid: 27431356 |
| [2] | 赵明磊, 毕齐. 缺血性脑卒中与短暂性脑缺血发作的二级预防[J]. 中华全科医师杂志, 2016, 15(3):167-169. |
| [3] |
Pajewski NM, Berlowitz DR, Bress AP, et al. Blood pressure thresholds and neurologic outcomes after endovascular therapy for acute ischemic stroke: An analysis of individual patient data from 3 randomized clinical trials[J]. JAMA Neurol, 2020, 77(5):622-631.
doi: 10.1001/jamaneurol.2019.4838 URL |
| [4] |
Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control[J]. N Engl J Med, 2015, 373(22):2103-2116.
doi: 10.1056/NEJMoa1511939 URL |
| [5] |
Gueyffi EF, Boissel JP, Boutitie F, et al. Effect of antihypertensive treatment in patients having already suffered from stroke. Gathering the evidence. The INDANA (INdividual Data ANalysis of Antihypertensive intervention trials) Project Collaborators[J]. Stroke, 1997, 28(12):2557-2562.
doi: 10.1161/01.STR.28.12.2557 URL |
| [6] |
Liu L, Wang Z, Gong L, et al. Blood pressure reduction for the secondary prevention of stroke: A Chinese trial and a systematic review of the literature[J]. Hypertens Res, 2009, 32(11):1032-1040.
doi: 10.1038/hr.2009.139 URL |
| [7] | 中国高血压防治指南修订委员会. 中国高血压防治指南2018年修订版[J]. 中国心血管杂志, 2019, 24(1):24-56. |
| [8] | Carey RM, Whelton PK. 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline[J]. Ann Intern Med, 2018, 168(5):351-358. |
| [9] | Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020)[EB/OL]. Cochrane, 2020. Available from www.training.cochrane.org/handbook. |
| [10] | PATS Collaborating Group. Post-stroke antihypertensive treatment study. A preliminary result[J]. Chin Med J (Engl), 1995, 108(9):710-717. |
| [11] | Mant J, McManus R, Roalfe A, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack:PAST-BP(Prevention After Stroke-Blood Pressure) randomised controlled trial[J]. BMJ, 2016, 352:i708. |
| [12] |
Hypertension-Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence[J]. JAMA, 1974, 229(4):409-418.
doi: 10.1001/jama.1974.03230420021019 URL |
| [13] |
The Dutch TIA Trial Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke[J]. Stroke, 1993, 24(4):543-548.
doi: 10.1161/01.STR.24.4.543 URL |
| [14] |
Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Sleight P, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients[J]. N Engl J Med, 2000, 342(3):145-153.
doi: 10.1056/NEJM200001203420301 URL |
| [15] |
PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure- lowering regimen among 6, 105 individuals with previous stroke or transient ischaemic attack[J]. Lancet, 2001, 358(9287):1033-1041.
doi: 10.1016/S0140-6736(01)06178-5 URL |
| [16] |
Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events[J]. N Engl J Med, 2008, 359(12):1225-1237.
doi: 10.1056/NEJMoa0804593 URL |
| [17] |
Pearce LA, McClure LA, Anderson DC, et al. Blood-pressure targets in patients with recent lacunar stroke: The SPS3 randomised trial[J]. Lancet, 2013, 382(9891):507-515.
doi: 10.1016/S0140-6736(13)60852-1 URL |
| [18] | Bath PM, Scutt P, Blackburn DJ, et al. Intensive versus guideline blood pressure and lipid lowering in patients with previous stroke: Main results from the pilot 'prevention of decline in cognition after stroke trial' (PODCAST) Randomised Controlled Trial[J]. PLoS One, 2017, 12(1):e164608. |
| [19] |
Kitagawa K, Yamamoto Y, Arima H, et al. Effect of standard vs intensive blood pressure control on the risk of recurrent stroke a randomized clinical trial and meta-analysis[J]. JAMA Neurol, 2019, 76(11):1309-1318.
doi: 10.1001/jamaneurol.2019.2167 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||