Clinical Focus ›› 2023, Vol. 38 ›› Issue (6): 493-499.doi: 10.3969/j.issn.1004-583X.2023.06.002
Previous Articles Next Articles
Values of IL-6 and CRP in predicting the severity of coronavirus disease 2019: A meta-analysis
Ni Yiyun1( ), Liu Bin1, Liang Qi2, Li Xiaofeng3
), Liu Bin1, Liang Qi2, Li Xiaofeng3
- 1. The Central Hospital of Yongzhou, Yongzhou, Yongzhou 425000, China
2. Shenzhen Hospital of Southern Medical University, Shenzhen 518000, China
3. Changsha Central Hospital, Changsha 410000, China
-
Received:2022-08-23Online:2023-06-20Published:2023-08-18 -
Contact:Ni Yiyun, Email:1538200160@qq.com
CLC Number:
Cite this article
Ni Yiyun, Liu Bin, Liang Qi, Li Xiaofeng. Values of IL-6 and CRP in predicting the severity of coronavirus disease 2019: A meta-analysis[J]. Clinical Focus, 2023, 38(6): 493-499.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2023.06.002
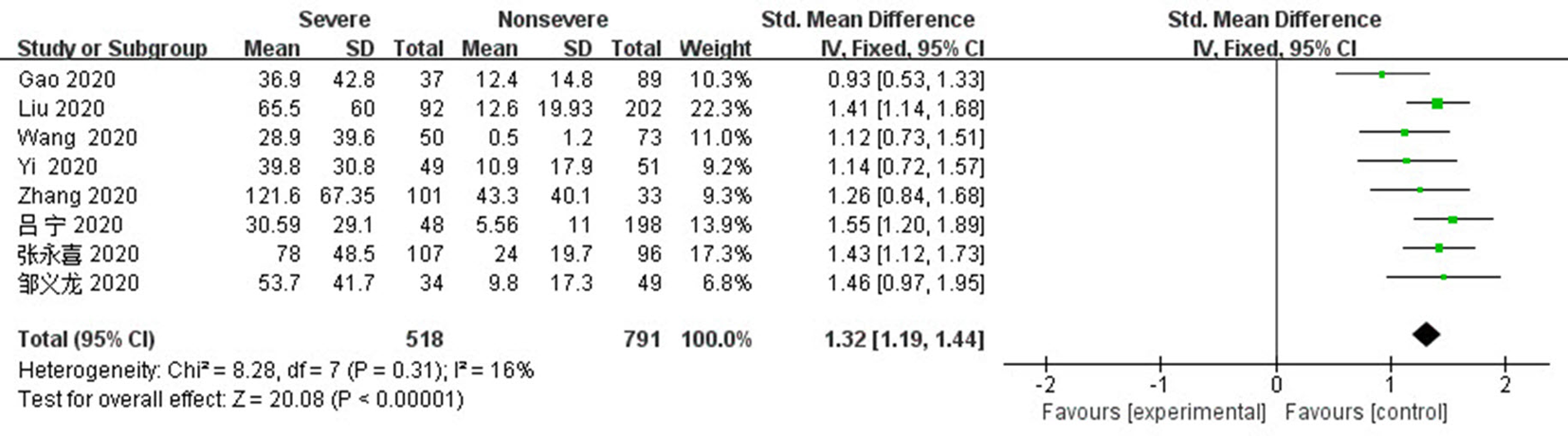
| 纳入研究 | 研究类型 | 研究来源 | 总样本量 | 性别 (男/女) | 平均数/中位数 年龄(岁) | NOS评分 |
|---|---|---|---|---|---|---|
| Liu 2020[ | 单中心回顾性研究 | 中国,武汉 | 294 | 162/132 | 56(39~67) | 6 |
| Jurado 2020[ | 多中心回顾性研究 | 西班牙,多个地区 | 584 | 349/235 | 63.0±16.5 | 8 |
| Yi 2020[ | 单中心回顾性研究 | 中国,浙江 | 100 | 63/37 | 54(42~64) | 6 |
| Zhang 2020[ | 单中心回顾性研究 | 中国,武汉 | 134 | 87/47 | 60.8±13.0 | 8 |
| Yuan 2020[ | 单中心回顾性研究 | 中国,福建 | 117 | 56/61 | 66(57~71) | 6 |
| Wei 2020[ | 单中心回顾性研究 | 中国,武汉 | 565 | 289/276 | 66(59~72) | 6 |
| Liao 2020[ | 多中心回顾性研究 | 中国,武汉 | 294 | 156/138 | 64(53~73) | 8 |
| Gao 2020[ | 单中心回顾性研究 | 中国,黑龙江 | 126 | 66/60 | 64.9±12.8 | 6 |
| Shi 2020[ | 多中心回顾性研究 | 中国,陕西 | 134 | 65/69 | 46(34~58) | 8 |
| Lv 2020[ | 单中心回顾性研究 | 中国,武汉 | 270 | 135/135 | 62.0±11.2 | 6 |
| Wang 2020[ | 单中心回顾性研究 | 中国,武汉 | 123 | 60/63 | 68(56~78) | 8 |
| 孙昀 2020[ | 多中心回顾性研究 | 中国,安徽 | 168 | 95/73 | 42.6±15.8 | 6 |
| 杨秀红 2020[ | 单中心回顾性研究 | 中国,武汉 | 365 | 162/203 | 60.2±14.2 | 8 |
| 魏剑浩 2020[ | 多中心回顾性研究 | 中国,上海 | 328 | 170/158 | 51.0±12.0 | 6 |
| 吕宁 2020[ | 单中心回顾性研究 | 中国,深圳 | 246 | 119/127 | 44.0±13.2 | 6 |
| 张永喜 2020[ | 单中心回顾性研究 | 中国,武汉 | 203 | 108/95 | 54.0±11.8 | 6 |
| 邹义龙 2020[ | 单中心回顾性研究 | 中国,武汉 | 83 | 36/47 | 67.0±12.9 | 6 |
| 刘浩 2020[ | 单中心回顾性研究 | 中国,武汉 | 152 | 84/68 | 56.5±13.5 | 6 |
Tab. 1 Basic characteristics and quality evaluation of the eligible literature
| 纳入研究 | 研究类型 | 研究来源 | 总样本量 | 性别 (男/女) | 平均数/中位数 年龄(岁) | NOS评分 |
|---|---|---|---|---|---|---|
| Liu 2020[ | 单中心回顾性研究 | 中国,武汉 | 294 | 162/132 | 56(39~67) | 6 |
| Jurado 2020[ | 多中心回顾性研究 | 西班牙,多个地区 | 584 | 349/235 | 63.0±16.5 | 8 |
| Yi 2020[ | 单中心回顾性研究 | 中国,浙江 | 100 | 63/37 | 54(42~64) | 6 |
| Zhang 2020[ | 单中心回顾性研究 | 中国,武汉 | 134 | 87/47 | 60.8±13.0 | 8 |
| Yuan 2020[ | 单中心回顾性研究 | 中国,福建 | 117 | 56/61 | 66(57~71) | 6 |
| Wei 2020[ | 单中心回顾性研究 | 中国,武汉 | 565 | 289/276 | 66(59~72) | 6 |
| Liao 2020[ | 多中心回顾性研究 | 中国,武汉 | 294 | 156/138 | 64(53~73) | 8 |
| Gao 2020[ | 单中心回顾性研究 | 中国,黑龙江 | 126 | 66/60 | 64.9±12.8 | 6 |
| Shi 2020[ | 多中心回顾性研究 | 中国,陕西 | 134 | 65/69 | 46(34~58) | 8 |
| Lv 2020[ | 单中心回顾性研究 | 中国,武汉 | 270 | 135/135 | 62.0±11.2 | 6 |
| Wang 2020[ | 单中心回顾性研究 | 中国,武汉 | 123 | 60/63 | 68(56~78) | 8 |
| 孙昀 2020[ | 多中心回顾性研究 | 中国,安徽 | 168 | 95/73 | 42.6±15.8 | 6 |
| 杨秀红 2020[ | 单中心回顾性研究 | 中国,武汉 | 365 | 162/203 | 60.2±14.2 | 8 |
| 魏剑浩 2020[ | 多中心回顾性研究 | 中国,上海 | 328 | 170/158 | 51.0±12.0 | 6 |
| 吕宁 2020[ | 单中心回顾性研究 | 中国,深圳 | 246 | 119/127 | 44.0±13.2 | 6 |
| 张永喜 2020[ | 单中心回顾性研究 | 中国,武汉 | 203 | 108/95 | 54.0±11.8 | 6 |
| 邹义龙 2020[ | 单中心回顾性研究 | 中国,武汉 | 83 | 36/47 | 67.0±12.9 | 6 |
| 刘浩 2020[ | 单中心回顾性研究 | 中国,武汉 | 152 | 84/68 | 56.5±13.5 | 6 |
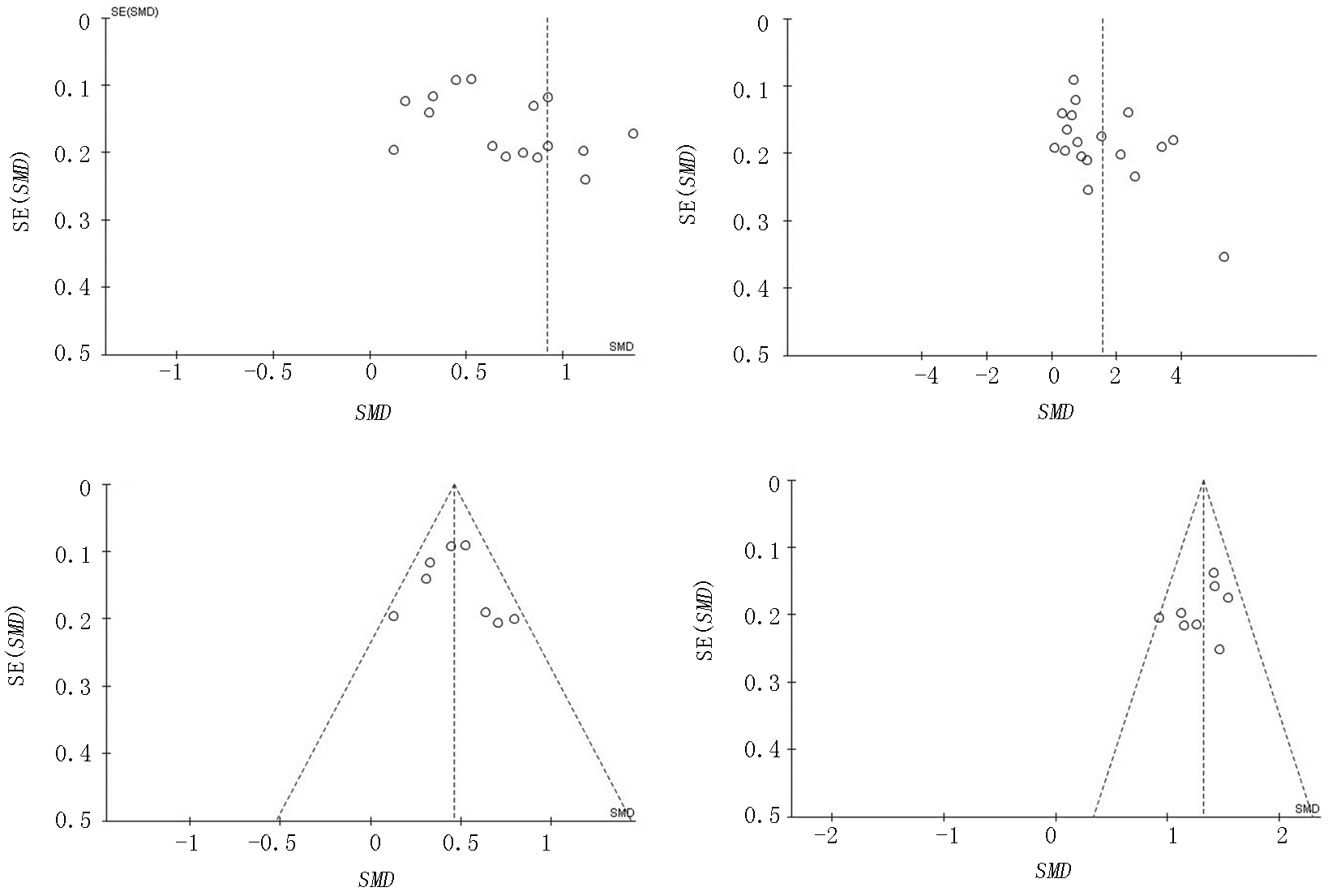
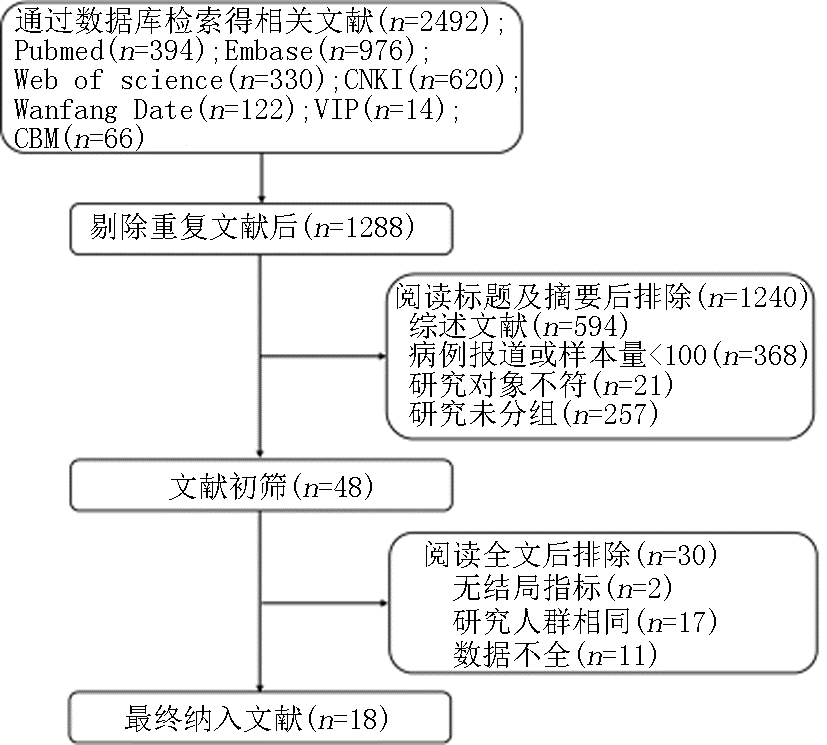
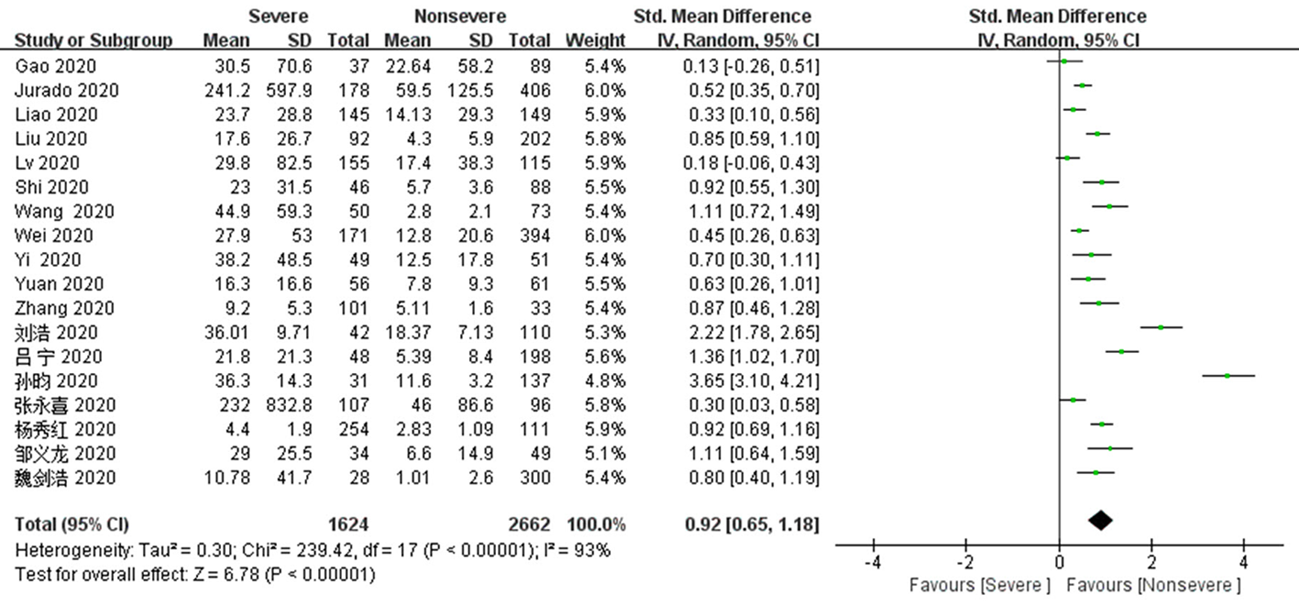
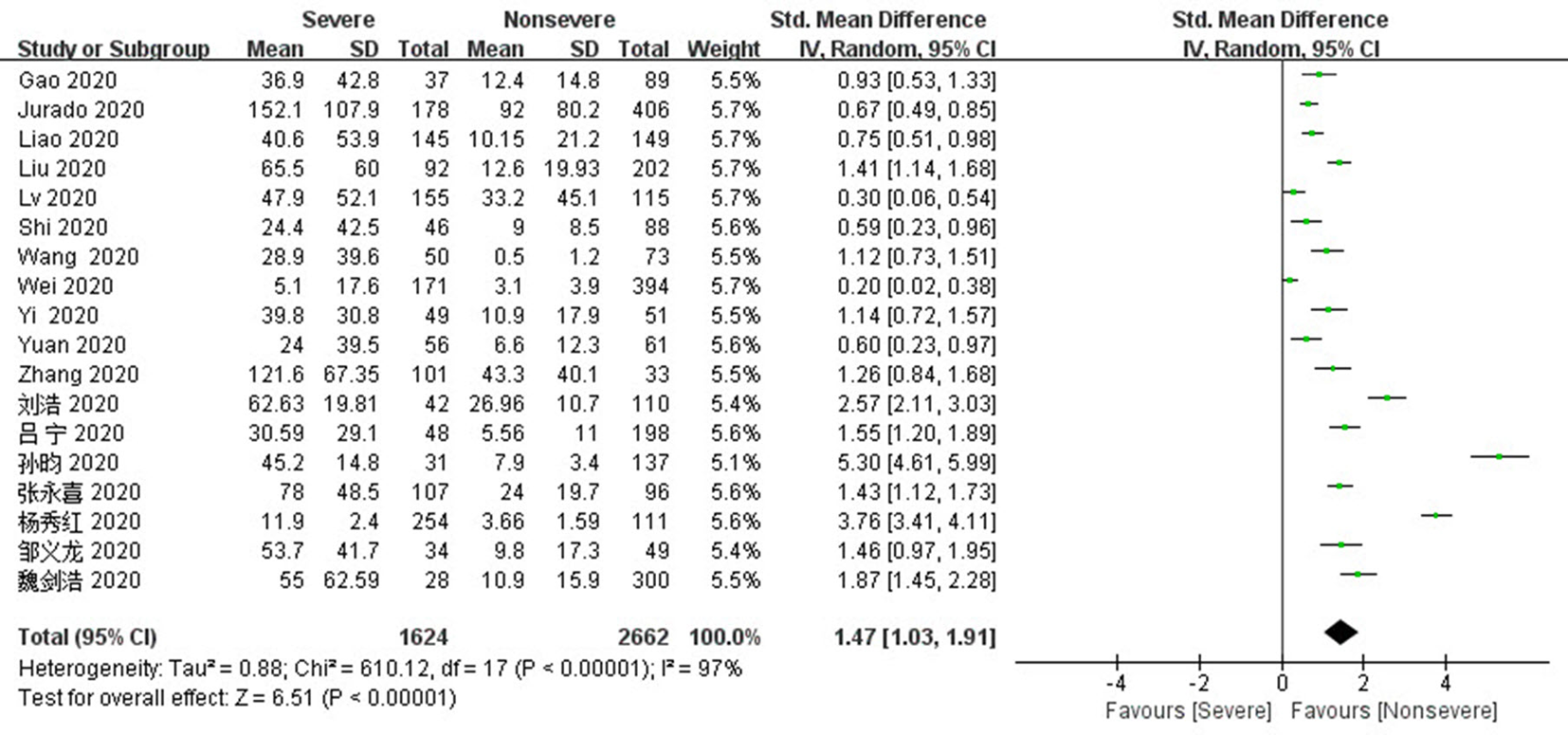
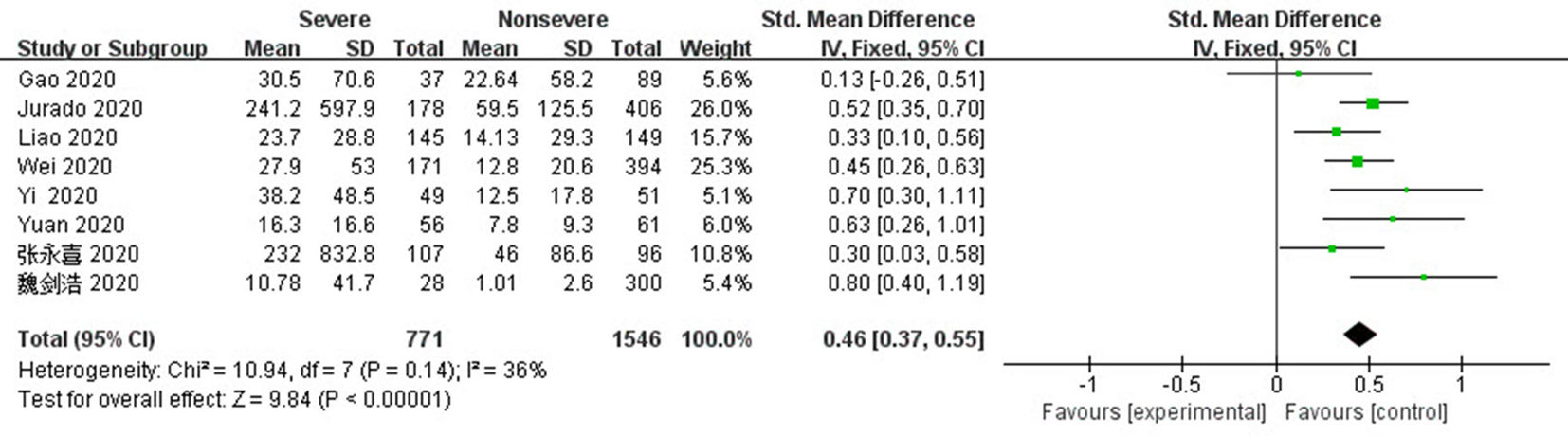
Fig. 6 Funnel plots of IL-6 and CRP levels of the severe group versus the mild group a,b:diagram corresponds to IL-6 and CRP respectively; c,d:diagram corresponds to IL-6 and CRP except heterogeneous literature
| [1] | World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[R]. 2020. |
| [2] | Coronavirus COVID-19 Global Cases[EB/OL]. https://covid19.who.int/.2021-04-09. |
| [3] | World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected., Interim Guidance[EB/OL]. https://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf. 2021-04-09. |
| [4] | 中华人民共和国国家卫生健康委员会. 关于印发新型冠状病毒肺炎诊疗方案(试行第八版)[EB/OL]. http://www.gov.cn/zhengce/zhengceku/202008/19/5535757/files/da89edf7cc9244fbb34ecf6c61df40bf.pdf. |
| [5] |
Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China[J]. N Engl J Med, 2020, 382(18):1708-1720.
doi: 10.1056/NEJMoa2002032 URL |
| [6] | Liao XL, Chen H, Li Z, et al. Critical care for severe coronavirus disease 2019: a population-based study from a province with low case-fatality rate in China[J]. Chin Med J (Engl), 2020, 134(1):98-100. |
| [7] | Wu R, Ai S, Cai J, et al. Predictive model and risk factors for case fatality of COVID-19: A cohort of 21, 392 cases in Hubei, China[J]. Innovation (Camb), 2020, 1(2):100022. |
| [8] |
Moore JB, June CH. Cytokine release syndrome in severe COVID-19[J]. Science, 2020, 368(6490):473-474.
doi: 10.1126/science.abb8925 pmid: 32303591 |
| [9] |
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome[J]. Lancet Respir Med, 2020, 8(4):420-422.
doi: 10.1016/S2213-2600(20)30076-X pmid: 32085846 |
| [10] |
Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab[J]. Proc Natl Acad Sci U S A, 2020, 117(20):10970-10975.
doi: 10.1073/pnas.2005615117 URL |
| [11] |
Campins L, Boixeda R, Perez-Cordon L, et al. Early tocilizumab treatment could improve survival among COVID-19 patients[J]. Clin Exp Rheumatol, 2020, 38(3):578.
pmid: 32456769 |
| [12] |
Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study[J]. Lancet Rheumatol, 2020, 2(8):e474-e484.
doi: 10.1016/S2665-9913(20)30173-9 URL |
| [13] |
Jurado A, Martín MC, Abad-Molina C, et al. COVID-19: age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study[J]. Immun Ageing, 2020, 17:22.
doi: 10.1186/s12979-020-00194-w pmid: 32802142 |
| [14] |
Del VD, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival[J]. Nat Med, 2020, 26(10):1636-1643.
doi: 10.1038/s41591-020-1051-9 |
| [15] |
Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19[J]. J Clin Virol, 2020, 127:104370.
doi: 10.1016/j.jcv.2020.104370 URL |
| [16] |
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses[J]. Eur J Epidemiol, 2010, 25(9):603-605.
doi: 10.1007/s10654-010-9491-z pmid: 20652370 |
| [17] |
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample[J]. BMC Med Res Methodol, 2005, 5:13.
pmid: 15840177 |
| [18] |
Liu L, Zheng Y, Cai L, et al. Neutrophil-to-lymphocyte ratio, a critical predictor for assessment of disease severity in patients with COVID-19[J]. Int J Lab Hematol, 2021, 43(2): 329-335.
doi: 10.1111/ijlh.13374 pmid: 33099889 |
| [19] |
Jurado A, Martín MC, Abad-Molina C, et al. COVID-19: Age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study[J]. Immun Ageing, 2020, 17:22.
doi: 10.1186/s12979-020-00194-w pmid: 32802142 |
| [20] |
Ping Y, Xiang Y, Cheng D, et al. Risk factors and clinical features of deterioration in COVID-19 patients in Zhejiang, China: a single-centre, retrospective study[J]. BMC Infect Dis, 2020, 20(1):943.
doi: 10.1186/s12879-020-05682-4 pmid: 33302889 |
| [21] |
Zhang L, Huang B, Xia H, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases[J]. Epidemiol Infect, 2020, 148:e199.
doi: 10.1017/S0950268820002010 URL |
| [22] |
Xiaohong Y, Wanling H, Bing Y, et al. Changes of hematological and immunological parameters in COVID-19 patients[J]. Int J Hematol, 2020, 112 (4):553-559.
doi: 10.1007/s12185-020-02930-w pmid: 32656638 |
| [23] |
Xiuqi W, Wenjuan Z, Jingyu S, et al. Hypolipidemia is associated with the severity of COVID-19[J]. J Clin Lipidol, 2020, 14(3) :297-304.
doi: S1933-2874(20)30078-7 pmid: 32430154 |
| [24] |
Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study[J]. Lancet Haematol, 2020, 7(9):e671-e678.
doi: 10.1016/S2352-3026(20)30217-9 pmid: 32659214 |
| [25] |
Yang G, Changsong W, Kai K, et al. Cytokine storm may not be the chief culprit for the deterioration of COVID-19.[J]. Viral Immunol, 2020, 34(5):336-341.
doi: 10.1089/vim.2020.0243 URL |
| [26] |
Shi P, Ren G, Yang J, et al. Clinical characteristics of imported and second-generation coronavirus disease 2019 (COVID-19) cases in Shaanxi outside Wuhan, China: A multicentre retrospective study[J]. Epidemiol Infect, 2020, 148:e238.
doi: 10.1017/S0950268820002332 URL |
| [27] |
Lv Z, Cheng S, Le J, et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: A retrospective cohort study[J]. Microbes Infect, 2020, 22(4-5):195-199.
doi: S1286-4579(20)30085-X pmid: 32425649 |
| [28] | Weili W, Zhongxiu Z, Xi L, et al. Clinical features and potential risk factors for discerning the critical cases and predicting the outcome of patients with COVID-19[J]. J Clin Lab Anal, 2020, 34(10):e23547. |
| [29] | 孙昀, 孙伟, 叶珺, 等. 168例新型冠状病毒肺炎患者临床特点及重症进展的影响因素分析[J]. 中华急诊医学杂志, 2020, 29(7):901-907. |
| [30] | 杨秀红, 熊蓉, 胡述立, 等. 不同临床分型新型冠状病毒肺炎患者临床特征及抗体、核酸检测结果分析[J]. 实用心脑肺血管病杂志, 2020, 28(9):10-15. |
| [31] | 魏剑浩, 郭倩, 李海聪, 等. 上海地区328例新型冠状病毒肺炎患者实验室数据分析[J]. 检验医学, 2020, 35(8):778-783. |
| [32] | 吕宁, 陆加刚, 罗莎莎, 等. 生化及炎症指标在新型冠状病毒肺炎患者中的变化特征和应用价值[J]. 国际检验医学杂志, 2020, 41(20):2501-2505. |
| [33] | 张永喜, 熊勇, 李新宇, 等. 新型冠状病毒肺炎出院患者203例的临床特点分析[J]. 中华传染病杂志, 2020, 38(8):472-478. |
| [34] | 邹义龙, 余贻汉, 刘尧蓓, 等. 新型冠状病毒肺炎患者病情严重程度与临床特征的关系[J]. 广西医学, 2020, 42(13):1707-1712. |
| [35] | 刘浩, 龙利, 张继波, 等. 新型冠状病毒肺炎患者血清胱抑素C与炎性因子水平变化及其相关性[J]. 天津医药, 2020, 48(8):753-756. |
| [36] |
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China[J]. JAMA, 2020, 323(11):1061-1069.
doi: 10.1001/jama.2020.1585 pmid: 32031570 |
| [37] |
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study[J]. Lancet, 2020, 395(10229):1054-1062.
doi: S0140-6736(20)30566-3 pmid: 32171076 |
| [38] | Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study[J]. BMJ, 2020, 369:m1966. |
| [39] |
Wang Y, Lu X, Li Y, et al. Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19[J]. Am J Respir Crit Care Med, 2020, 201(11):1430-1434.
doi: 10.1164/rccm.202003-0736LE URL |
| [40] |
Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19[J]. Clin Infect Dis, 2021, 73(2):e445-e454.
doi: 10.1093/cid/ciaa954 pmid: 32651997 |
| [41] |
Potere N, Di Nisio M, Cibelli D, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: A case-control study[J]. Ann Rheum Dis, 2021, 80(2):1-2.
doi: 10.1136/annrheumdis-2020-218243 pmid: 32647027 |
| [42] |
Price CC, Altice FL, Shyr Y, et al. Tocilizumab Treatment for Cytokine Release Syndrome in Hospitalized Patients With Coronavirus Disease 2019: Survival and Clinical Outcomes[J]. Chest, 2020, 158(4):1397-1408.
doi: 10.1016/j.chest.2020.06.006 pmid: 32553536 |
| [43] |
Jurado A, Martín MC, Abad-Molina C, et al. COVID-19: age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study[J]. Immun Ageing, 2020, 17:22.
doi: 10.1186/s12979-020-00194-w pmid: 32802142 |
| [44] |
Potere N, Di Nisio M, Cibelli D, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case-control study[J]. Ann Rheum Dis, 2021, 80(2):1-2.
doi: 10.1136/annrheumdis-2020-218243 pmid: 32647027 |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 50
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 195
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||