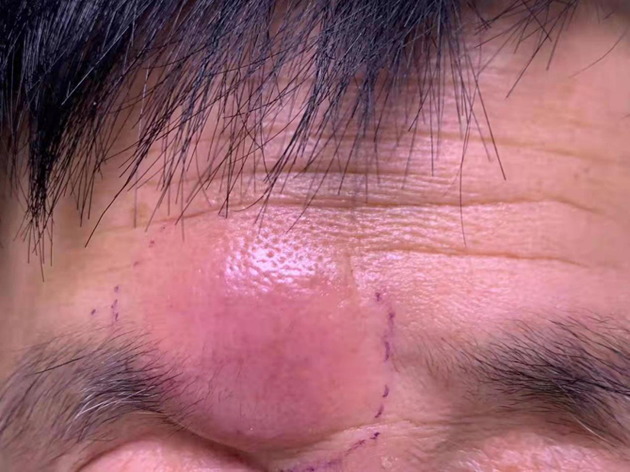
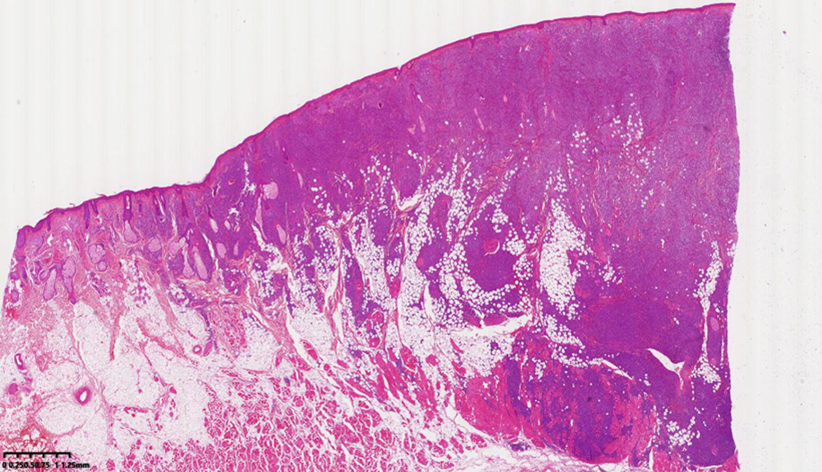
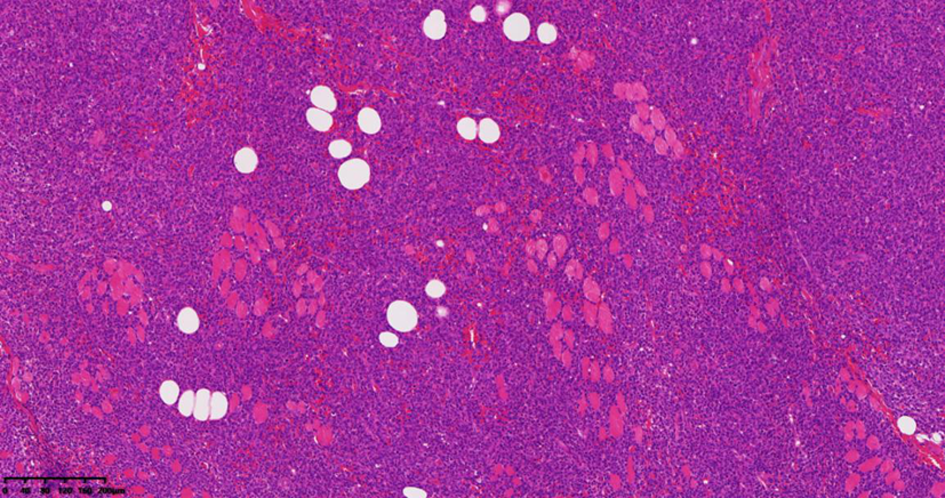
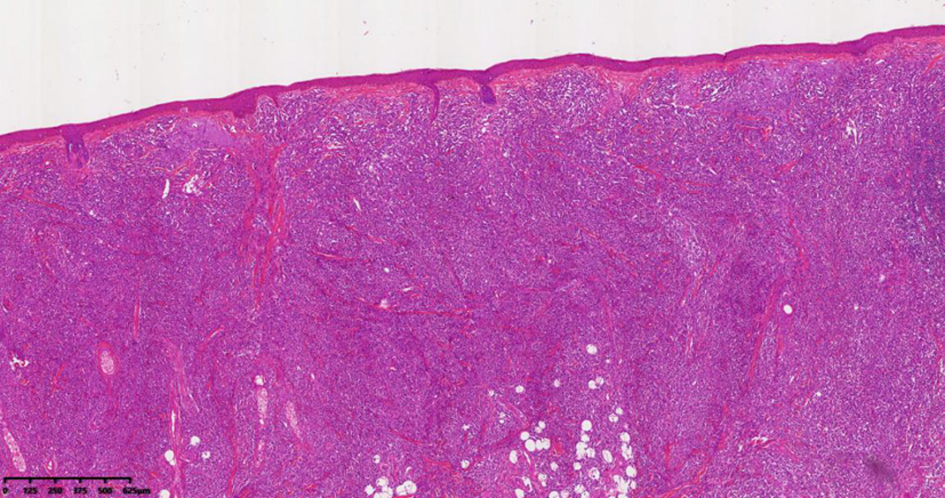
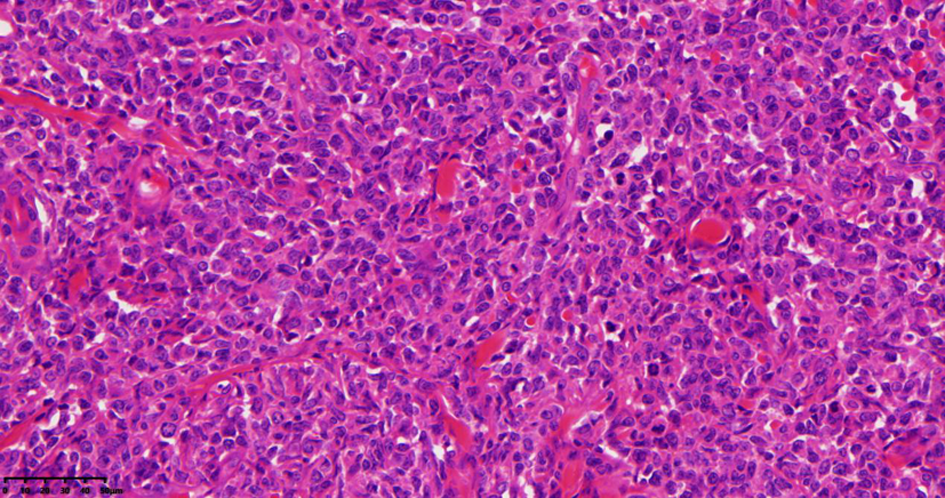
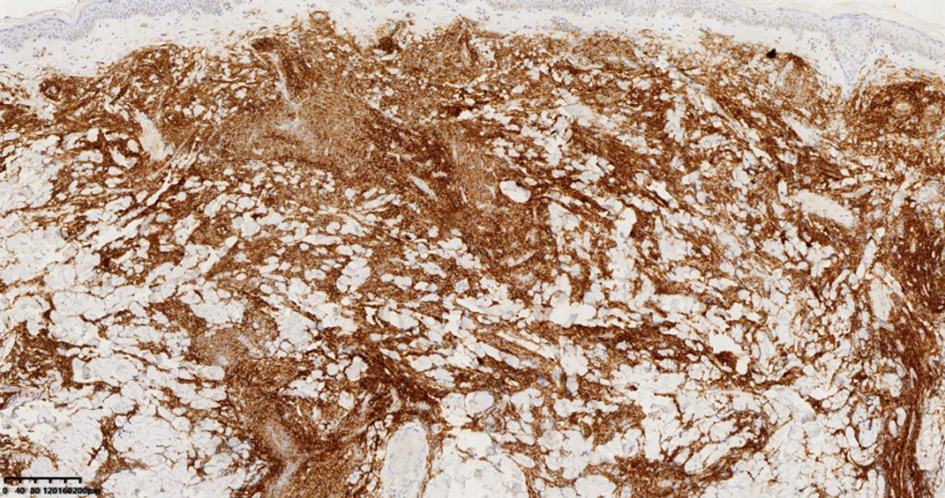
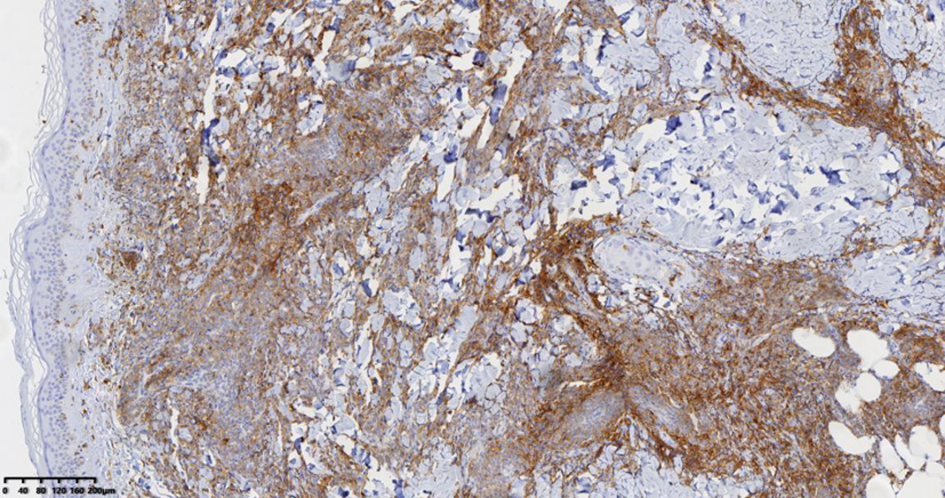
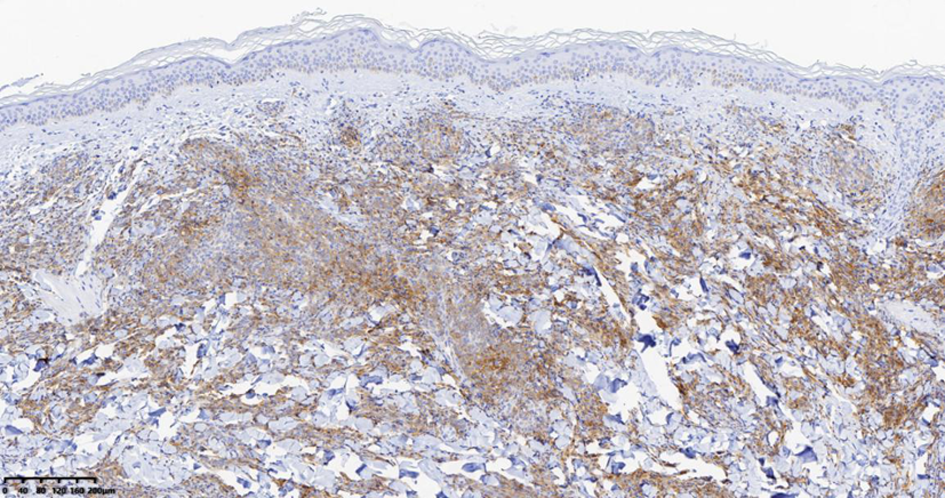
| [1] |
邱录贵. 2008年版WHO淋巴组织肿瘤分类的新变化[J]. 中华血液学杂志, 2009, 30(7):433-434.
|
| [2] |
Zhang Y, Xiong J, Li S, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm: A retrospective study of morphological and immunophenotypic features[J]. Eur J Dermatol, 2022, 32(1): 70-76.
doi: 10.1684/ejd.2022.4195
pmid: 35171796
|
| [3] |
Prasad T, Pushpam D, Chopra A. Cytomorphological and immunophenotypic characteristics of blastic plasmacytoid dendritic cell neoplasm involving central nervous system: A case report and review of literature[J]. Cytopathology, 2022, 33(4): 522-529.
|
| [4] |
王峰蓉, 许兰平. 母细胞性浆细胞样树突细胞肿瘤诊断及治疗进展[J]. 中华血液学杂志, 2016, 37(1):75-78.
|
| [5] |
陈晓君, 刘彦权, 黄素蓉, 等. 母细胞性浆细胞样树突细胞肿瘤的研究进展[J]. 解放军医学杂志, 2021, 46(10):1040-1044.
|
| [6] |
王维娜, 齐硕, 王娅南. 睾丸母细胞性浆细胞样树突细胞肿瘤一例[J]. 中华病理学杂志, 2019, 48(9):730-732.
|
| [7] |
Pemmaraju N, Wilson NR, Garcia-Manero G, et al. Characteristics and outcomes of patients with blastic plasmacytoid dendritic cell neoplasm treated with frontline HCVAD[J]. Blood Adv, 2022, 6(10): 3027-3035.
|
| [8] |
Yin CC, Pemmaraju N, You MJ, et al. Integrated clinical genotype-phenotype characteristics of blastic plasmacytoid dendritic cell neoplasm[J]. Cancers (Basel), 2021, 13(23): 5888.
|
| [9] |
Sapienza MR, Abate F, Melle F, et al. Blastic plasmacytoid dendritic cell neoplasm: Genomics mark epigenetic dysregulation as a primary therapeutic target[J]. Haematologica, 2019, 104(4): 729-737.
doi: 10.3324/haematol.2018.202093
pmid: 30381297
|
| [10] |
林兰, 罗序亮, 徐静, 等. 母细胞性浆细胞样树突细胞肿瘤发病机制与治疗的研究进展[J]. 临床与实验病理学杂志, 2019, 35(11):1330-1333.
|
| [11] |
张悦昕, 陈喜雪. 自行消退的皮肤母细胞性浆细胞样树突细胞肿瘤[J]. 临床皮肤科杂志, 2019, 48(10):612-615.
|
| [12] |
张茂功, 董欣, 杨朋. 母细胞性浆细胞样树突细胞肿瘤[J]. 临床皮肤科杂志, 2022, 51(2):67+65-66.
|
| [13] |
Ulrickson ML, Puri A, Lindstrom S, et al. Gemcitabine and docetaxel as a novel treatment regimen for blastic plasmacytoid dendritic cell neoplasm[J]. Am J Hematol, 2017, 92(5): E75-E77.
|
 )
)