Clinical Focus ›› 2022, Vol. 37 ›› Issue (11): 985-991.doi: 10.3969/j.issn.1004-583X.2022.11.004
Previous Articles Next Articles
The correlation between the prognosis of lung adenocarcinoma with acquired resistance to the first-generation EGFR TKI and the T790M mutation
Li Shufan1, Wang Yuxiu2, Jiang Zhenghua2( )
)
- 1. Yangzhou Hongquan Hospital,Yangzhou 225200,China
2. Department of Respiratory and Critical Care Medicine,North Jiangsu People's Hospital,Yangzhou 225000,China
-
Received:2022-07-25Online:2022-11-20Published:2023-01-02 -
Contact:Jiang Zhenghua E-mail:yzjzhhua@163.com
CLC Number:
Cite this article
Li Shufan, Wang Yuxiu, Jiang Zhenghua. The correlation between the prognosis of lung adenocarcinoma with acquired resistance to the first-generation EGFR TKI and the T790M mutation[J]. Clinical Focus, 2022, 37(11): 985-991.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2022.11.004
| 临床特征 | T790M阴性 ( | T790M阳性 ( | χ2/ | |
|---|---|---|---|---|
| 性别 | ||||
| 女 男 | 9(60.0) 6(40.0) | 42(53.8) 36(46.2) | 0.192 | 0.661 |
| 年龄(岁) | ||||
| <65 ≥65 | 11(73.3) 4(26.7) | 44(56.4) 34(43.6) | 1.491 | 0.222 |
| 吸烟状态 | ||||
| 不吸烟 吸烟 | 11(73.3) 4(26.7) | 57(73.1) 21(26.9) | 0 | 0.984 |
| ECOG评分 | ||||
| 0 | 2(13.3) | 7(9.0) | ||
| 1 | 5(33.3) | 47(60.3) | 4.119 | 0.249 |
| 2 | 7(46.7) | 19(24.4) | ||
| 3 | 5(6.7) | 1(6.4) | ||
| 分期 | ||||
| 术后复发 初诊Ⅳ期 | 3(20.0) 12(80.0) | 16(20.5) 62(79.5) | 0.002 | 0.964 |
| 肿瘤大小 | ||||
| <5 cm ≥5 cm | 7(46.7) 8(53.3) | 39(50.0) 39(50.0) | 0.056 | 0.813 |
| 淋巴结状态 | ||||
| N0 | 2(13.3) | 19(24.4) | ||
| N1-2 | 6(40.0) | 36(46.2) | 1.934 | 0.380 |
| N3 | 7(46.7) | 23(29.5) | ||
| 外显子突变 | ||||
| 19外显子 21外显子 | 6(40.0) 9(60.0) | 47(60.3) 31(39.7) | 2.106 | 0.147 |
| 奥希替尼治疗线数 | ||||
| 二线 三线及三线以上 | 3(20.0) 12(80.0) | 39(50.0) 39(50.0) | 4.572 | 0.033 |
| 临床特征 | T790M阴性 ( | T790M阳性 ( | χ2/ | |
|---|---|---|---|---|
| 性别 | ||||
| 女 男 | 9(60.0) 6(40.0) | 42(53.8) 36(46.2) | 0.192 | 0.661 |
| 年龄(岁) | ||||
| <65 ≥65 | 11(73.3) 4(26.7) | 44(56.4) 34(43.6) | 1.491 | 0.222 |
| 吸烟状态 | ||||
| 不吸烟 吸烟 | 11(73.3) 4(26.7) | 57(73.1) 21(26.9) | 0 | 0.984 |
| ECOG评分 | ||||
| 0 | 2(13.3) | 7(9.0) | ||
| 1 | 5(33.3) | 47(60.3) | 4.119 | 0.249 |
| 2 | 7(46.7) | 19(24.4) | ||
| 3 | 5(6.7) | 1(6.4) | ||
| 分期 | ||||
| 术后复发 初诊Ⅳ期 | 3(20.0) 12(80.0) | 16(20.5) 62(79.5) | 0.002 | 0.964 |
| 肿瘤大小 | ||||
| <5 cm ≥5 cm | 7(46.7) 8(53.3) | 39(50.0) 39(50.0) | 0.056 | 0.813 |
| 淋巴结状态 | ||||
| N0 | 2(13.3) | 19(24.4) | ||
| N1-2 | 6(40.0) | 36(46.2) | 1.934 | 0.380 |
| N3 | 7(46.7) | 23(29.5) | ||
| 外显子突变 | ||||
| 19外显子 21外显子 | 6(40.0) 9(60.0) | 47(60.3) 31(39.7) | 2.106 | 0.147 |
| 奥希替尼治疗线数 | ||||
| 二线 三线及三线以上 | 3(20.0) 12(80.0) | 39(50.0) 39(50.0) | 4.572 | 0.033 |
| 临床特征 | 例数 | T790M阴性 ( | T790M阳性 ( | χ2值 | |
|---|---|---|---|---|---|
| 所有人群ORR | |||||
| CR+PR SD+PD | 50(53.7) 43(46.3) | 2(13.3) 13(86.7) | 48(61.5) 30(38.5) | 11.760 | 0.001 |
| 二线ORR | |||||
| CR+PR SD+PD | 26(61.9) 16(38.1) | 0(0.0) 3(100.0) | 26(66.7) 13(33.3) | 5.250 | 0.022 |
| 三线ORR | |||||
| CR+PR SD+PD | 24(47.1) 27(52.9) | 2(16.7) 10(83.3) | 22(56.4) 17(43.6) | 5.818 | 0.016 |
| 所有人群DCR | |||||
| CR+PR+SD PD | 87(93.5) 6(6.5) | 11(73.3) 4(6.4) | 76(97.4) 2(2.6) | 12.109 | 0.01 |
| 二线DCR | |||||
| CR+PR+SD PD | 40(95.2) 2(4.8) | 2(66.7) 1(33.3) | 38(97.4) 1(2.6) | 5.815 | 0.016 |
| 三线DCR | |||||
| CR+PR+SD PD | 50(53.7) 4(7.8) | 9(75.0) 3(25.0) | 38(97.4) 1(2.6) | 6.391 | 0.011 |
| 临床特征 | 例数 | T790M阴性 ( | T790M阳性 ( | χ2值 | |
|---|---|---|---|---|---|
| 所有人群ORR | |||||
| CR+PR SD+PD | 50(53.7) 43(46.3) | 2(13.3) 13(86.7) | 48(61.5) 30(38.5) | 11.760 | 0.001 |
| 二线ORR | |||||
| CR+PR SD+PD | 26(61.9) 16(38.1) | 0(0.0) 3(100.0) | 26(66.7) 13(33.3) | 5.250 | 0.022 |
| 三线ORR | |||||
| CR+PR SD+PD | 24(47.1) 27(52.9) | 2(16.7) 10(83.3) | 22(56.4) 17(43.6) | 5.818 | 0.016 |
| 所有人群DCR | |||||
| CR+PR+SD PD | 87(93.5) 6(6.5) | 11(73.3) 4(6.4) | 76(97.4) 2(2.6) | 12.109 | 0.01 |
| 二线DCR | |||||
| CR+PR+SD PD | 40(95.2) 2(4.8) | 2(66.7) 1(33.3) | 38(97.4) 1(2.6) | 5.815 | 0.016 |
| 三线DCR | |||||
| CR+PR+SD PD | 50(53.7) 4(7.8) | 9(75.0) 3(25.0) | 38(97.4) 1(2.6) | 6.391 | 0.011 |
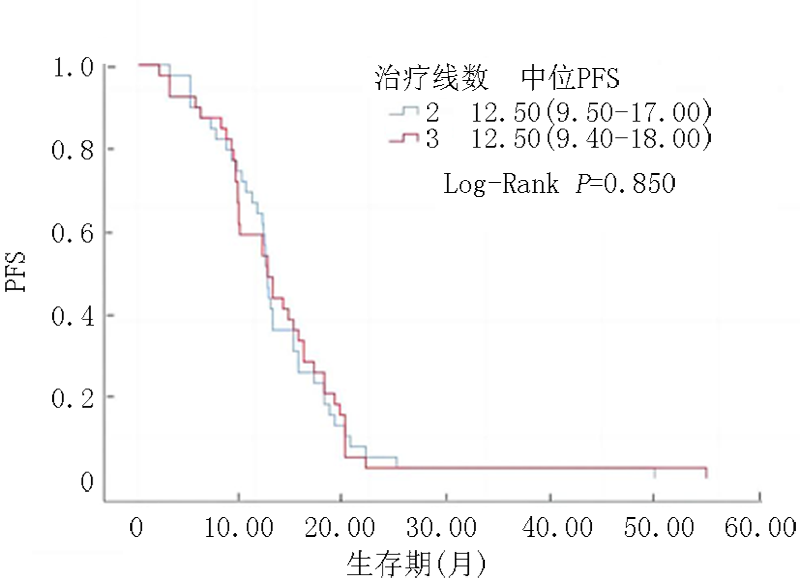
| PFS | T790M阴性 ( | T790M阳性 ( | ||
|---|---|---|---|---|
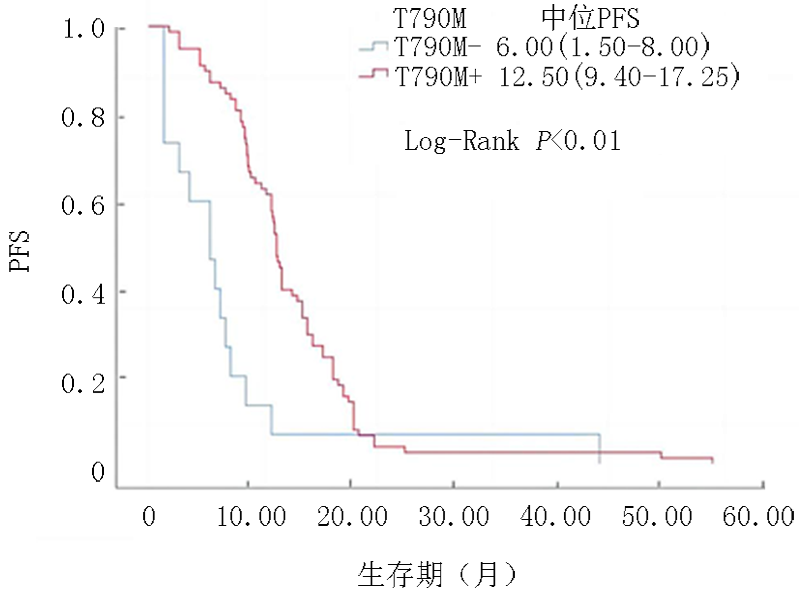
| 所有人群 | 6.0(1.5~8.0) | 12.5(9.4~17.3) | -4.155 | <0.01 |
| 二线 | 6.5(1.5~8.0) ( | 12.5(9.5~17.0) ( | -2.150 | 0.022 |
| 三线 | 6.0(1.9~7.9) ( | 12.5(9.4~18.0) ( | -3.322 | 0.016 |
| PFS | T790M阴性 ( | T790M阳性 ( | ||
|---|---|---|---|---|
| 所有人群 | 6.0(1.5~8.0) | 12.5(9.4~17.3) | -4.155 | <0.01 |
| 二线 | 6.5(1.5~8.0) ( | 12.5(9.5~17.0) ( | -2.150 | 0.022 |
| 三线 | 6.0(1.9~7.9) ( | 12.5(9.4~18.0) ( | -3.322 | 0.016 |
| 变量 | 单因素分析 | 多因素分析 | |||
|---|---|---|---|---|---|
| HR(95% | HR(95% | ||||
| 性别 | |||||
| 女 | 1.00 | ||||
| 男 | 1.083 (0.717~1.634) | 0.706 | |||
| 年龄(岁) | |||||
| <65 | 1.00 | ||||
| ≥65 | 1.346 (0.881~2.057) | 0.170 | |||
| 吸烟状态 | |||||
| 不吸烟 | 1.00 | ||||
| 吸烟 | 1.076 (0.667~1.710) | 0.757 | |||
| ECOG评分 | |||||
| 0 | 1.00 | ||||
| 1 | 0.447 (0.152~1.317) | 0.144 | |||
| 2 | 0.485 (0.206~1.142) | 0.098 | |||
| 3 | 0.591 (0.241~1.448) | 0.250 | |||
| 分期 | 0.724 (0.218~2.401) | 0.597 | |||
| 术后复发 | 1.00 | ||||
| 初诊Ⅳ期 | 1.128 (0.670~1.899) | 0.650 | |||
| 肿瘤大小 | |||||
| <5 cm | 1.00 | 1.00 | |||
| ≥5 cm | 0.661 (0.433~1.010) | 0.056 | 0.575 (0.373~0.885) | 0.012 | |
| 淋巴结状态 | |||||
| N0 | 1.00 | ||||
| N1~2 | 0.946 (0.536~1.669) | 0.848 | |||
| N3 | 1.229 (0.762~1.983) | 0.398 | |||
| 外显子突变 | |||||
| 19外显子 | 1.00 | 1.00 | |||
| 21外显子 | 0.580 (0.378~0.890) | 0.013 | 0.551 (0.357~0.853) | 0.007 | |
| 奥希替尼治疗线数 | |||||
| 二线 | 1.00 | ||||
| 三线及三线以上 | 0.950 (0.630~1.433) | 0.806 | |||
| T790M状态 | |||||
| T790M- | 1.00 | 1.00 | |||
| T790M+ | 2.747 (1.545~4.886) | 0.01 | 2.972 (1.643~5.379) | <0.01 | |
| 变量 | 单因素分析 | 多因素分析 | |||
|---|---|---|---|---|---|
| HR(95% | HR(95% | ||||
| 性别 | |||||
| 女 | 1.00 | ||||
| 男 | 1.083 (0.717~1.634) | 0.706 | |||
| 年龄(岁) | |||||
| <65 | 1.00 | ||||
| ≥65 | 1.346 (0.881~2.057) | 0.170 | |||
| 吸烟状态 | |||||
| 不吸烟 | 1.00 | ||||
| 吸烟 | 1.076 (0.667~1.710) | 0.757 | |||
| ECOG评分 | |||||
| 0 | 1.00 | ||||
| 1 | 0.447 (0.152~1.317) | 0.144 | |||
| 2 | 0.485 (0.206~1.142) | 0.098 | |||
| 3 | 0.591 (0.241~1.448) | 0.250 | |||
| 分期 | 0.724 (0.218~2.401) | 0.597 | |||
| 术后复发 | 1.00 | ||||
| 初诊Ⅳ期 | 1.128 (0.670~1.899) | 0.650 | |||
| 肿瘤大小 | |||||
| <5 cm | 1.00 | 1.00 | |||
| ≥5 cm | 0.661 (0.433~1.010) | 0.056 | 0.575 (0.373~0.885) | 0.012 | |
| 淋巴结状态 | |||||
| N0 | 1.00 | ||||
| N1~2 | 0.946 (0.536~1.669) | 0.848 | |||
| N3 | 1.229 (0.762~1.983) | 0.398 | |||
| 外显子突变 | |||||
| 19外显子 | 1.00 | 1.00 | |||
| 21外显子 | 0.580 (0.378~0.890) | 0.013 | 0.551 (0.357~0.853) | 0.007 | |
| 奥希替尼治疗线数 | |||||
| 二线 | 1.00 | ||||
| 三线及三线以上 | 0.950 (0.630~1.433) | 0.806 | |||
| T790M状态 | |||||
| T790M- | 1.00 | 1.00 | |||
| T790M+ | 2.747 (1.545~4.886) | 0.01 | 2.972 (1.643~5.379) | <0.01 | |
| [1] |
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018[J]. CA Cancer J Clin, 2018, 68(1):7-30.
doi: 10.3322/caac.21442 URL |
| [2] |
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6):394-424.
doi: 10.3322/caac.21492 URL |
| [3] |
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR[J]. N Engl J Med, 2010, 362(25):2380-2388.
doi: 10.1056/NEJMoa0909530 URL |
| [4] |
Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial[J]. JAMA, 2003, 290(16):2149-2158.
doi: 10.1001/jama.290.16.2149 pmid: 14570950 |
| [5] |
Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib[J]. N Engl J Med, 2005, 352(8):786-792.
doi: 10.1056/NEJMoa044238 URL |
| [6] |
Campo M, Gerber D, Gainor JF, et al. Acquired resistance to first-Line afatinib and the challenges of prearranged progression biopsies[J]. J Thorac Oncol, 2016, 11(11):2022-2026.
doi: S1556-0864(16)30846-2 pmid: 27553514 |
| [7] |
Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer[J]. Cancer Discov, 2014, 4(9):1046-1061.
doi: 10.1158/2159-8290.CD-14-0337 pmid: 24893891 |
| [8] |
Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): A phase 3 randomised trial[J]. Lancet Oncol, 2015, 16(8):990-998.
doi: 10.1016/S1470-2045(15)00121-7 URL |
| [9] |
Mok T, Kim SW, Wu YL, et al. Gefitinib plus chemotherapy versus chemotherapy in epidermal growth factor receptor mutation-positive non-small-cell lung cancer resistant to first-line gefitinib (IMPRESS): Overall survival and biomarker analyses[J]. J Clin Oncol, 2017, 35(36):4027-4034.
doi: 10.1200/JCO.2017.73.9250 pmid: 28968167 |
| [10] |
Goldman JW, Noor ZS, Remon J, et al. Are liquid biopsies a surrogate for tissue EGFR testing?[J]. Ann Oncol, 2018, 29(suppl_1):i38-i46.
doi: 10.1093/annonc/mdx706 URL |
| [11] |
Wang Z, Chen R, Wang S, et al. Quantification and dynamic monitoring of EGFR T790M in plasma cell-free DNA by digital PCR for prognosis of EGFR-TKI treatment in advanced NSCLC[J]. PLoS One, 2014, 9(11):e110780.
doi: 10.1371/journal.pone.0110780 URL |
| [12] |
Papadimitrakopoulou VA, Han JY, Ahn MJ, et al. Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: Osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer[J]. Cancer, 2020, 126(2):373-380.
doi: 10.1002/cncr.32503 pmid: 31769875 |
| [13] |
Mok TS, Wu Y, Ahn M, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-Positive lung cancer[J]. N Engl J Med, 2017, 376(7):629-640.
doi: 10.1056/NEJMoa1612674 URL |
| [14] |
Marinis F, Wu YL, de Castro GJ, et al. ASTRIS: a global real-world study of osimertinib in >3000 patients with EGFR T790M positive non-small-cell lung cancer[J]. Future Oncol, 2019, 15(26):3003-3014.
doi: 10.2217/fon-2019-0324 URL |
| [15] |
Mu Y, Xing P, Hao X, et al. Real-world data of osimertinib in patients with pretreated non-small cell lung cancer: A retrospective study[J]. Cancer Manag Res, 2019, 11:9243-9251.
doi: 10.2147/CMAR.S221434 pmid: 31802944 |
| [16] |
Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer[J]. N Engl J Med, 2015, 372(18):1689-1699.
doi: 10.1056/NEJMoa1411817 URL |
| [17] |
Eide I, Helland A, Ekman S, et al. Osimertinib in T790M-positive and -negative patients with EGFR-mutated advanced non-small cell lung cancer (the TREM-study)[J]. Lung Cancer, 2020, 143:27-35.
doi: S0169-5002(20)30336-6 pmid: 32200138 |
| [18] |
Hong S, Gao F, Fu S, et al. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-mutant advanced non-small cell lung cancer[J]. JAMA Oncol, 2018, 4(5):739-742.
doi: 10.1001/jamaoncol.2018.0049 pmid: 29596544 |
| [19] | Zhang Z, Zhang Y, Luo F, et al. Dual blockade of EGFR and VEGFR pathways: Results from a pilot study evaluating apatinib plus gefitinib as a first-line treatment for advanced EGFR-mutant non-small cell lung cancer[J]. Clin Transl Med, 2020, 10(2):e33. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||