Clinical Focus ›› 2021, Vol. 36 ›› Issue (3): 220-224.doi: 10.3969/j.issn.1004-583X.2021.03.006
Previous Articles Next Articles
Clinical value of CEA, CA125, and CYFRA21-1 levels in efficacy evaluation of EGFR-TKIs in the treatment of EGFR mutation-positive non-small cell lung adenocarcinoma
- 1. Department of Oncology, Tinglin Hospital, Jinshan District, Shanghai 201505, China
2. Department of Oncology, Jinshan Hospital of Fudan University, Shanghai 201508, China
-
Received:2021-01-26Online:2021-03-20Published:2021-03-29 -
Contact:Qiao Tiankui E-mail:qiaotk@163.com
CLC Number:
Cite this article
He Yiwen, Qiao Tiankui. Clinical value of CEA, CA125, and CYFRA21-1 levels in efficacy evaluation of EGFR-TKIs in the treatment of EGFR mutation-positive non-small cell lung adenocarcinoma[J]. Clinical Focus, 2021, 36(3): 220-224.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2021.03.006
| 项目 | 例数 | 中位PFS(月) | Z值 | P值 | 中位OS(月) | Z值 | P值 |
|---|---|---|---|---|---|---|---|
| 年龄(岁) | |||||||
| ≤70 >70 | 158 28 | 8.9 10.3 | 0.793 | 0.489 | 22.8 23.9 | 0.821 | 0.437 |
| 性别 | |||||||
| 男 女 | 79 107 | 10.7 8.5 | 0.877 | 0.413 | 23.7 22.0 | 0.363 | 0.816 |
| PS评分 | |||||||
| 0~1 2~4 | 157 29 | 9.8 4.9 | 4.249 | <0.01 | 23.7 9.4 | 5.824 | <0.01 |
| 临床分期 | |||||||
| Ⅲb Ⅳ | 11 175 | 9.2 8.6 | 0.611 | 0.523 | 23.5 22.7 | 0.482 | 0.724 |
| EGFR基因 | |||||||
| 19外显子缺失突变 | 107 | 8.8 | 23.7 | ||||
| 21外显子错义突变 | 72 | 7.9 | 0.923 | 0.384 | 19.4 | 1.034 | 0.202 |
| 其他 | 7 | 9.3 | 20.2 | ||||
| EGFR-TKIs治疗 | |||||||
| 埃克替尼 | 109 | 8.9 | 20.8 | ||||
| 吉非替尼 | 51 | 9.6 | 0.284 | 0.941 | 22.5 | 0.862 | 0.433 |
| 厄洛替尼 | 26 | 10.8 | 23.1 | ||||
| 远处转移 | |||||||
| 是 否 | 65 121 | 6.9 11.0 | 4.146 | <0.01 | 13.8 25.9 | 4.724 | <0.01 |
| CEA水平(ng/ml) | |||||||
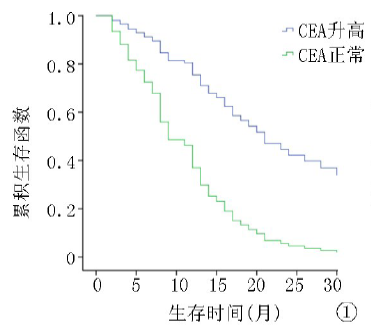
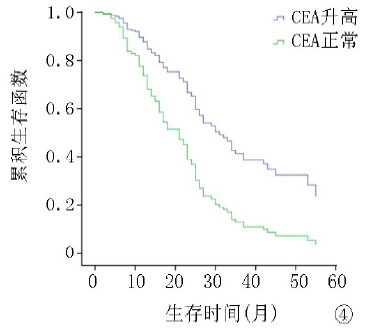
| ≤5 >5 | 64 122 | 8.1 13.2 | 4.071 | <0.01 | 12.8 26.7 | 5.643 | <0.01 |
| CA125水平(U/ml) | |||||||
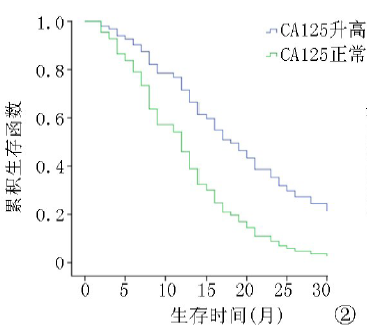
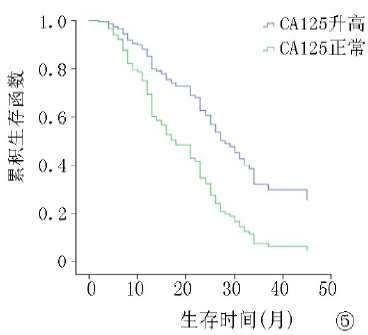
| ≤35 >35 | 57 129 | 8.2 13.5 | 3.954 | <0.01 | 12.4 26.8 | 5.923 | <0.01 |
| CYFRA21-1(ng/ml) | |||||||
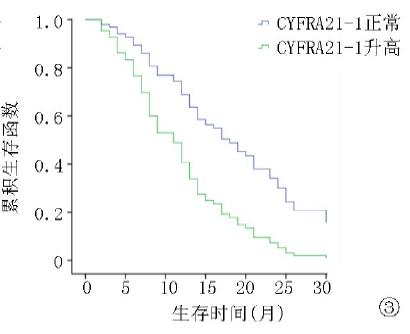
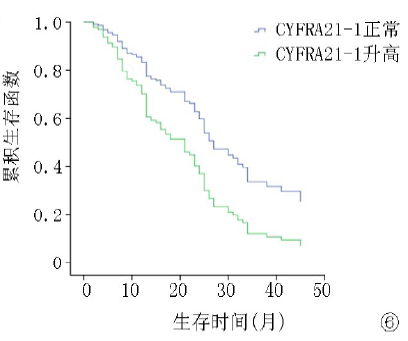
| ≤3.3 >3.3 | 105 81 | 12.8 8.3 | 4.672 | <0.01 | 25.8 13.5 | 5.028 | <0.01 |
| 项目 | 例数 | 中位PFS(月) | Z值 | P值 | 中位OS(月) | Z值 | P值 |
|---|---|---|---|---|---|---|---|
| 年龄(岁) | |||||||
| ≤70 >70 | 158 28 | 8.9 10.3 | 0.793 | 0.489 | 22.8 23.9 | 0.821 | 0.437 |
| 性别 | |||||||
| 男 女 | 79 107 | 10.7 8.5 | 0.877 | 0.413 | 23.7 22.0 | 0.363 | 0.816 |
| PS评分 | |||||||
| 0~1 2~4 | 157 29 | 9.8 4.9 | 4.249 | <0.01 | 23.7 9.4 | 5.824 | <0.01 |
| 临床分期 | |||||||
| Ⅲb Ⅳ | 11 175 | 9.2 8.6 | 0.611 | 0.523 | 23.5 22.7 | 0.482 | 0.724 |
| EGFR基因 | |||||||
| 19外显子缺失突变 | 107 | 8.8 | 23.7 | ||||
| 21外显子错义突变 | 72 | 7.9 | 0.923 | 0.384 | 19.4 | 1.034 | 0.202 |
| 其他 | 7 | 9.3 | 20.2 | ||||
| EGFR-TKIs治疗 | |||||||
| 埃克替尼 | 109 | 8.9 | 20.8 | ||||
| 吉非替尼 | 51 | 9.6 | 0.284 | 0.941 | 22.5 | 0.862 | 0.433 |
| 厄洛替尼 | 26 | 10.8 | 23.1 | ||||
| 远处转移 | |||||||
| 是 否 | 65 121 | 6.9 11.0 | 4.146 | <0.01 | 13.8 25.9 | 4.724 | <0.01 |
| CEA水平(ng/ml) | |||||||
| ≤5 >5 | 64 122 | 8.1 13.2 | 4.071 | <0.01 | 12.8 26.7 | 5.643 | <0.01 |
| CA125水平(U/ml) | |||||||
| ≤35 >35 | 57 129 | 8.2 13.5 | 3.954 | <0.01 | 12.4 26.8 | 5.923 | <0.01 |
| CYFRA21-1(ng/ml) | |||||||
| ≤3.3 >3.3 | 105 81 | 12.8 8.3 | 4.672 | <0.01 | 25.8 13.5 | 5.028 | <0.01 |
| 变量 | 赋值 | 变量 | 赋值 |
|---|---|---|---|
| 年龄 | ≤70岁=0;>70=1 | EGFR-TKIs治疗 | 埃克替尼=1;吉非替尼=2;厄洛替尼=3 |
| 性别 | 男=0;女=1 | 是否远处转移 | 否=0;是=1; |
| PS评分 | 0~1分=0;2~4分=1 | CEA水平 | ≤5 ng/ml=0;>5 ng/ml=1 |
| 临床分期 | Ⅲb=0;Ⅳ=1 | CA125水平 | ≤35 U/ml=0;>35 U/ml=1 |
| EGFR基因 | 19外显子缺失突变=1;21外显子错义突变=2;其他=3 | CYFRA21-1水平 | ≤3.3 ng/ml=0;>3.3 ng/ml=1 |
| 变量 | 赋值 | 变量 | 赋值 |
|---|---|---|---|
| 年龄 | ≤70岁=0;>70=1 | EGFR-TKIs治疗 | 埃克替尼=1;吉非替尼=2;厄洛替尼=3 |
| 性别 | 男=0;女=1 | 是否远处转移 | 否=0;是=1; |
| PS评分 | 0~1分=0;2~4分=1 | CEA水平 | ≤5 ng/ml=0;>5 ng/ml=1 |
| 临床分期 | Ⅲb=0;Ⅳ=1 | CA125水平 | ≤35 U/ml=0;>35 U/ml=1 |
| EGFR基因 | 19外显子缺失突变=1;21外显子错义突变=2;其他=3 | CYFRA21-1水平 | ≤3.3 ng/ml=0;>3.3 ng/ml=1 |
| 变量 | 回归系数 | 标准误 | Wald χ2值 | P值 | OR值 | 95%CI | |
|---|---|---|---|---|---|---|---|
| 下限 | 上限 | ||||||
| PS评分(2~4分) | 1.956 | 0.523 | 17.853 | <0.01 | 5.753 | 2.538 | 9.838 |
| CEA水平 | -1.256 | 0.424 | 9.824 | <0.01 | 3.964 | 1.163 | 5.637 |
| CA125水平 | -1.442 | 0.432 | 12.532 | <0.01 | 4.234 | 1.545 | 5.853 |
| CYFRA21-1水平 | 1.895 | 0.495 | 14.828 | <0.01 | 4.533 | 1.824 | 7.725 |
| 变量 | 回归系数 | 标准误 | Wald χ2值 | P值 | OR值 | 95%CI | |
|---|---|---|---|---|---|---|---|
| 下限 | 上限 | ||||||
| PS评分(2~4分) | 1.956 | 0.523 | 17.853 | <0.01 | 5.753 | 2.538 | 9.838 |
| CEA水平 | -1.256 | 0.424 | 9.824 | <0.01 | 3.964 | 1.163 | 5.637 |
| CA125水平 | -1.442 | 0.432 | 12.532 | <0.01 | 4.234 | 1.545 | 5.853 |
| CYFRA21-1水平 | 1.895 | 0.495 | 14.828 | <0.01 | 4.533 | 1.824 | 7.725 |
| 变量 | 回归系数 | 标准误 | Wald χ2值 | P值 | OR值 | 95%CI | |
|---|---|---|---|---|---|---|---|
| 下限 | 上限 | ||||||
| PS评分(2~4分) | 1.351 | 0.517 | 13.728 | <0.01 | 4.728 | 2.042 | 7.265 |
| CEA水平 | -0.937 | 0.485 | 6.628 | 0.005 | 2.343 | 1.042 | 4.274 |
| CA125水平 | -1.043 | 0.524 | 9.292 | <0.01 | 3.792 | 1.143 | 4.544 |
| CYFRA21-1水平 | 1.194 | 0.497 | 11.372 | <0.01 | 4.164 | 1.156 | 6.622 |
| 变量 | 回归系数 | 标准误 | Wald χ2值 | P值 | OR值 | 95%CI | |
|---|---|---|---|---|---|---|---|
| 下限 | 上限 | ||||||
| PS评分(2~4分) | 1.351 | 0.517 | 13.728 | <0.01 | 4.728 | 2.042 | 7.265 |
| CEA水平 | -0.937 | 0.485 | 6.628 | 0.005 | 2.343 | 1.042 | 4.274 |
| CA125水平 | -1.043 | 0.524 | 9.292 | <0.01 | 3.792 | 1.143 | 4.544 |
| CYFRA21-1水平 | 1.194 | 0.497 | 11.372 | <0.01 | 4.164 | 1.156 | 6.622 |
| [1] |
Singal G, Miller PG, Agarwala V, et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database[J]. JAMA, 2019,321(14):1391-1399.
doi: 10.1001/jama.2019.3241 URL |
| [2] |
Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, Version 4. 2016[J]. J Natl Compr Canc Netw, 2016,14(3):255-264.
pmid: 26957612 |
| [3] |
Kim H, Jung HI, Kwon SH, et al. Preoperative neutrophil-lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis[J]. Ann Surg Treat Res, 2019,96(4):191-200.
doi: 10.4174/astr.2019.96.4.191 URL |
| [4] |
Sekiguchi H, Shimamoto K, Takano M, et al. Cancer antigen-125 plasma level as a biomarker of new-onset atrial fibrillation in postmenopausal women[J]. Heart, 2017,103(17):1368-1373.
doi: 10.1136/heartjnl-2016-310272 pmid: 28285269 |
| [5] | Sone K, Oguri T, Ito K, et al. Predictive role of CYFRA21-1 and CEA for subsequent docetaxel in non-small cell lung cancer patients[J]. Anticancer Res, 2017,37(9):5125-5127. |
| [6] | 于林楠, 张奎全, 祁啸, 等. miR-144-3p靶向EGFR对肺癌细胞迁移、侵袭和自噬的影响研究[J]. 中国免疫学杂志, 2019,35(20):2504-2508. |
| [7] | 朱永林, 黄瑛, 王金乐, 等. 沉默免疫负调控基因技术联合辅助化疗治疗结肠癌的回顾性分析[J]. 中国基层医药, 2018,25(24):3175-3179. |
| [8] |
Brückl W, Tufman A, Huber RM. Advanced non-small cell lung cancer (NSCLC) with activating EGFR mutations: first-line treatment with afatinib and other EGFR TKIs[J]. Expert Rev Anticancer Ther, 2017,17(2):143-155.
doi: 10.1080/14737140.2017.1266265 URL |
| [9] |
La MS, Cretella D, Bonelli M, et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFRT-KI osimertinib in NSCLC EGFR mutated cell lines[J]. J Exp Clin Cancer Res, 2017,36(1):174-176.
doi: 10.1186/s13046-017-0653-7 URL |
| [10] | Cacho DB, Spinola MH, Mendoza LG, et al. Association of neurologic manifestations and CEA levels with the diagnosis of brain metastases in lung cancer patients[J]. C1in Transl Oncol, 2019,21(11):1538. |
| [11] | 李标, 张有为. CRP、CEA及CA125表达水平与非小细胞肺癌患者行VATS术后疗效及预后的相关性[J]. 临床肺科杂志, 2020,25(5):724-729. |
| [12] | 卢畅, 申淑景, 毛玉焕, 等. CEA、 CYFRA21-1、 NSE水平与EGFR突变的晚期肺腺癌患者临床疗效的相关性[J]. 肿瘤防治研究, 2017,44(7):485-488. |
| [13] | 高原, 宋平平, 刘希斌, 等. 肺腺癌患者血清CEA水平与EGFR-TKIs疗效相关性分析[J]. 中华肿瘤防治杂志, 2016,23(9):601-604. |
| [14] | Fakhar HB, Rezaie TM, Zali H, et al. Comparison of serum human epididymis protein ( HE4), carbohydrate antigen 125(CA125) and risk of ovarian malignancy algorithm (ROMA) as markers in ovarian cancer:a systematic review and a meta-analysis[J]. Indian J Gynecol Oncol, 2018,16(1):10-15. |
| [15] | 段素华, 王素梅. 血清CYFRA21-1、CEA、CA125联合检测在非小细胞肺癌中的诊断价值[J]. 实用临床医药杂志, 2020,24(8):55-57, 62. |
| [16] | 谢冰峰, 黎明, 朱勇军, 等. 肺癌患者围化疗期血清CYFRA21-1、NSE和 CA125水平变化的临床价值[J]. 重庆医学, 2019,48(6):1049-1051. |
| [17] |
Sone K, Oguri T, Nakao M, et al. CYFRA 21-1 as a predictive marker for non-small cell lung cancer treated with pemetrexed-based chemotherapy[J]. Anticancer Res, 2017,37(2):935-937.
doi: 10.21873/anticanres URL |
| [18] | 顾军娟, 廖生俊, 陈雅文, 等. 肺癌患者血清CA125、CEA和CYFRA21-1水平检测价值分析[J]. 微循环学杂志, 2019,29(1):33-38. |
| [19] | 张利祥, 潘静, 赵洁, 等. 痰液基细胞学检查与血清CYFRA21-1, CEA, NSE联合检测对肺癌的诊断价值[J]. 现代检验医学杂志, 2019,34(3):99-103. |
| [20] |
Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer[J]. J Clin Oncol, 2018,36(9):841-849.
doi: 10.1200/JCO.2017.74.7576 URL |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||