Clinical Focus ›› 2023, Vol. 38 ›› Issue (7): 606-612.doi: 10.3969/j.issn.1004-583X.2023.07.004
Previous Articles Next Articles
Correlation of anti-phospholipase A2 receptor antibody with idiopathic membranous nephropathy
Wang Tao( ), Gao Yuwei, Wang Xinghua, Hu Xiuhong, Cui Hongrui, Xu Baozhen, Yang Hongjuan
), Gao Yuwei, Wang Xinghua, Hu Xiuhong, Cui Hongrui, Xu Baozhen, Yang Hongjuan
- Department of Nephrology, the First Hospital of Hebei Medical University, Shijiazhuang 050030, China
-
Received:2023-05-19Online:2023-07-20Published:2023-09-01 -
Contact:Wang Tao E-mail:757559650@qq.com
CLC Number:
Cite this article
Wang Tao, Gao Yuwei, Wang Xinghua, Hu Xiuhong, Cui Hongrui, Xu Baozhen, Yang Hongjuan. Correlation of anti-phospholipase A2 receptor antibody with idiopathic membranous nephropathy[J]. Clinical Focus, 2023, 38(7): 606-612.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2023.07.004
| 组别 | 例数 | 年龄 (岁) | 性别 (男/女) | 病程 (年) | 高血压 (%) | 吸烟 (%) | 尿红细胞计数 (/μl) | 24 h UTP (g/d) | TP (g/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA2R-Ab抗体阴性 | 27 | 49.36±13.93 | 21/6 | 4.49±1.49 | 12(44.44) | 10(37.04) | 14.22±3.47 | 3.47±1.75 | 45.82±4.51 | |||||
| PLA2R-Ab抗体阳性 | 62 | 52.48±16.27 | 45/17 | 3.76±2.05 | 34(54.84) | 32(51.61) | 19.57±8.94 | 5.34±2.18 | 40.24±5.74 | |||||
| t/χ2值 | 0.461 | 0.265 | 0.915 | 0.814 | 1.604 | 1.764 | 2.154 | 2.421 | ||||||
| P值 | 0.651 | 0.607 | 0.373 | 0.367 | 0.205 | 0.095 | 0.041 | 0.026 | ||||||
| 组别 | ALB (g/L) | TC (mmol/L) | TG (mmol/L) | BUN (mmol/L) | Scr (μmol/L) | eGFR [ml/(min·1.73 m2)] | Sys-C (mg/L) | |||||||
| PLA2R-Ab抗体阴性 | 26.47±3.49 | 8.36±3.25 | 2.87±1.67 | 5.22±2.48 | 67.45±18.24 | 83.63±18.38 | 1.15±0.32 | |||||||
| PLA2R-Ab抗体阳性 | 22.33±4.71 | 7.25±2.18 | 2.87±1.83 | 6.46±2.28 | 71.87±23.57 | 81.49±23.47 | 1.45±0.64 | |||||||
| t/χ2 | 2.235 | 0.897 | 0.681 | 1.173 | 0.468 | 0.226 | 1.334 | |||||||
| P值 | 0.038 | 0.381 | 0.548 | 0.256 | 0.644 | 0.823 | 0.198 | |||||||
Tab.1 Comparison of baseline data between groups
| 组别 | 例数 | 年龄 (岁) | 性别 (男/女) | 病程 (年) | 高血压 (%) | 吸烟 (%) | 尿红细胞计数 (/μl) | 24 h UTP (g/d) | TP (g/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA2R-Ab抗体阴性 | 27 | 49.36±13.93 | 21/6 | 4.49±1.49 | 12(44.44) | 10(37.04) | 14.22±3.47 | 3.47±1.75 | 45.82±4.51 | |||||
| PLA2R-Ab抗体阳性 | 62 | 52.48±16.27 | 45/17 | 3.76±2.05 | 34(54.84) | 32(51.61) | 19.57±8.94 | 5.34±2.18 | 40.24±5.74 | |||||
| t/χ2值 | 0.461 | 0.265 | 0.915 | 0.814 | 1.604 | 1.764 | 2.154 | 2.421 | ||||||
| P值 | 0.651 | 0.607 | 0.373 | 0.367 | 0.205 | 0.095 | 0.041 | 0.026 | ||||||
| 组别 | ALB (g/L) | TC (mmol/L) | TG (mmol/L) | BUN (mmol/L) | Scr (μmol/L) | eGFR [ml/(min·1.73 m2)] | Sys-C (mg/L) | |||||||
| PLA2R-Ab抗体阴性 | 26.47±3.49 | 8.36±3.25 | 2.87±1.67 | 5.22±2.48 | 67.45±18.24 | 83.63±18.38 | 1.15±0.32 | |||||||
| PLA2R-Ab抗体阳性 | 22.33±4.71 | 7.25±2.18 | 2.87±1.83 | 6.46±2.28 | 71.87±23.57 | 81.49±23.47 | 1.45±0.64 | |||||||
| t/χ2 | 2.235 | 0.897 | 0.681 | 1.173 | 0.468 | 0.226 | 1.334 | |||||||
| P值 | 0.038 | 0.381 | 0.548 | 0.256 | 0.644 | 0.823 | 0.198 | |||||||
| 组别 | 例数 | Ⅰ期 [例(%)] | Ⅱ期 [例(%)] | Ⅲ期 [例(%)] | 球性硬化 [例(%)] | 节段性硬化 [例(%)] | 新月体形成 [例(%)] | 肾小管间质 积分 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA2R-Ab抗体阴性 | 27 | 4(14.81) | 22(81.48) | 1(3.70) | 11(40.74) | 4(14.81) | 2(7.41) | 2.27±1.32 | ||||||
| PLA2R-Ab抗体阳性 | 62 | 11(17.74) | 48(77.42) | 3(4.84) | 26(41.94) | 8(12.91) | 5(8.06) | 1.95±1.11 | ||||||
| t/χ2值 | 0.307 | 0.651 | 0.068 | 0.589 | ||||||||||
| P值 | 0.579 | 0.798 | 0.793 | 0.563 | ||||||||||
| 组别 | 基底膜厚度 (nm) | IgG (g/L) | lgA (g/L) | IgM (g/L) | C3 (g/L) | C4 (g/L) | ||||||||
| PLA2R-Ab抗体阴性 | 1492.00±264.00 | 5.57±2.23 | 2.94±1.83 | 1.13±0.92 | 1.12±0.38 | 0.46±0.31 | ||||||||
| PLA2R-Ab抗体阳性 | 1078.00±436.00 | 6.63±2.71 | 2.31±1.02 | 1.37±0.83 | 1.44±0.57 | 0.37±0.28 | ||||||||
| t/χ2值 | 2.567 | 0.956 | 0.954 | 0.635 | 1.493 | 0.539 | ||||||||
| P值 | 0.019 | 0.351 | 0.329 | 0.531 | 0.153 | 0.596 | ||||||||
Tab.2 Comparison of renal pathological data between groups
| 组别 | 例数 | Ⅰ期 [例(%)] | Ⅱ期 [例(%)] | Ⅲ期 [例(%)] | 球性硬化 [例(%)] | 节段性硬化 [例(%)] | 新月体形成 [例(%)] | 肾小管间质 积分 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA2R-Ab抗体阴性 | 27 | 4(14.81) | 22(81.48) | 1(3.70) | 11(40.74) | 4(14.81) | 2(7.41) | 2.27±1.32 | ||||||
| PLA2R-Ab抗体阳性 | 62 | 11(17.74) | 48(77.42) | 3(4.84) | 26(41.94) | 8(12.91) | 5(8.06) | 1.95±1.11 | ||||||
| t/χ2值 | 0.307 | 0.651 | 0.068 | 0.589 | ||||||||||
| P值 | 0.579 | 0.798 | 0.793 | 0.563 | ||||||||||
| 组别 | 基底膜厚度 (nm) | IgG (g/L) | lgA (g/L) | IgM (g/L) | C3 (g/L) | C4 (g/L) | ||||||||
| PLA2R-Ab抗体阴性 | 1492.00±264.00 | 5.57±2.23 | 2.94±1.83 | 1.13±0.92 | 1.12±0.38 | 0.46±0.31 | ||||||||
| PLA2R-Ab抗体阳性 | 1078.00±436.00 | 6.63±2.71 | 2.31±1.02 | 1.37±0.83 | 1.44±0.57 | 0.37±0.28 | ||||||||
| t/χ2值 | 2.567 | 0.956 | 0.954 | 0.635 | 1.493 | 0.539 | ||||||||
| P值 | 0.019 | 0.351 | 0.329 | 0.531 | 0.153 | 0.596 | ||||||||
| 组别 | 例数 | ACEI/ARB的 使用 | 随访时间 | |||
|---|---|---|---|---|---|---|
| 3个月 | 6个月 | 9个月 | 12个月 | |||
| PLA2R-Ab抗体阴性 | 27 | 57(91.94) | 15(24.19) | 30(48.38) | 37(59.68) | 43(69.35) |
| PLA2R-Ab抗体阳性 | 62 | 24(88.89) | 11(40.74) | 14(51.85) | 19(70.37) | 24(88.89) |
| t/χ2值 | 0.035 | 2.491 | 0.093 | 0.922 | 3.856 | |
| P值 | 0.953 | 0.114 | 0.764 | 0.337 | 0.041 | |
Tab. 3 Comparison of follow-up outcomes between groups[n (%)]
| 组别 | 例数 | ACEI/ARB的 使用 | 随访时间 | |||
|---|---|---|---|---|---|---|
| 3个月 | 6个月 | 9个月 | 12个月 | |||
| PLA2R-Ab抗体阴性 | 27 | 57(91.94) | 15(24.19) | 30(48.38) | 37(59.68) | 43(69.35) |
| PLA2R-Ab抗体阳性 | 62 | 24(88.89) | 11(40.74) | 14(51.85) | 19(70.37) | 24(88.89) |
| t/χ2值 | 0.035 | 2.491 | 0.093 | 0.922 | 3.856 | |
| P值 | 0.953 | 0.114 | 0.764 | 0.337 | 0.041 | |
| 组别 | 例数 | 时间 | UTP (g/d) | ALB (g/L) | Scr (μmol/L) | PLA2R-Ab (RU/ml) |
|---|---|---|---|---|---|---|
| PLA2R-Ab抗体阴性 | 27 | 基线 | 3.47±1.75 | 25.05±4.83 | 60.07±17.12 | 7.65±5.31 |
| 3个月 | 1.66±1.08△ | 30.63±3.14*△ | 56.56±24.12 | 5.88±4.61 | ||
| 6个月 | 0.92±0.81△ | 35.37±8.11*△ | 54.68±23.96 | 8.31±4.28 | ||
| 12个月 | 0.63±0.48△ | 43.19±7.72*△ | 52.68±20.87 | 4.76±2.29 | ||
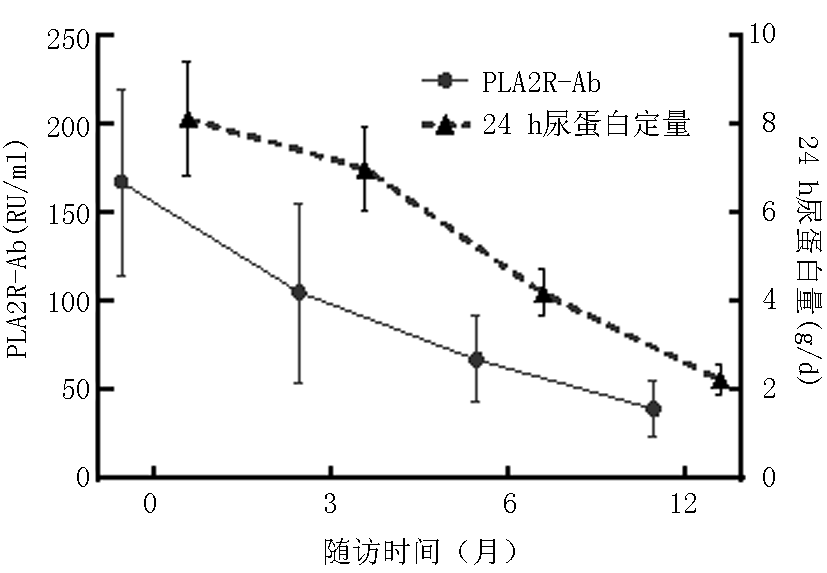
| PLA2R-Ab抗体阳性 | 62 | 基线 | 5.34±2.18 | 21.45±5.71 | 60.58±16.26 | 157.03±73.14 |
| 3个月 | 3.17±1.39 | 26.71±4.69 | 55.67±24.32 | 90.27±64.39△ | ||
| 6个月 | 2.52±1.63△ | 29.34±4.02△ | 53.95±22.28 | 73.68±40.69△ | ||
| 12个月 | 1.81±0.69△ | 34.57±7.29△ | 51.61±15.03 | 39.96±15.96△ |
Tab. 4 Comparison of clinical indicators and PLA2R antibody titers between groups( x -±s)
| 组别 | 例数 | 时间 | UTP (g/d) | ALB (g/L) | Scr (μmol/L) | PLA2R-Ab (RU/ml) |
|---|---|---|---|---|---|---|
| PLA2R-Ab抗体阴性 | 27 | 基线 | 3.47±1.75 | 25.05±4.83 | 60.07±17.12 | 7.65±5.31 |
| 3个月 | 1.66±1.08△ | 30.63±3.14*△ | 56.56±24.12 | 5.88±4.61 | ||
| 6个月 | 0.92±0.81△ | 35.37±8.11*△ | 54.68±23.96 | 8.31±4.28 | ||
| 12个月 | 0.63±0.48△ | 43.19±7.72*△ | 52.68±20.87 | 4.76±2.29 | ||
| PLA2R-Ab抗体阳性 | 62 | 基线 | 5.34±2.18 | 21.45±5.71 | 60.58±16.26 | 157.03±73.14 |
| 3个月 | 3.17±1.39 | 26.71±4.69 | 55.67±24.32 | 90.27±64.39△ | ||
| 6个月 | 2.52±1.63△ | 29.34±4.02△ | 53.95±22.28 | 73.68±40.69△ | ||
| 12个月 | 1.81±0.69△ | 34.57±7.29△ | 51.61±15.03 | 39.96±15.96△ |
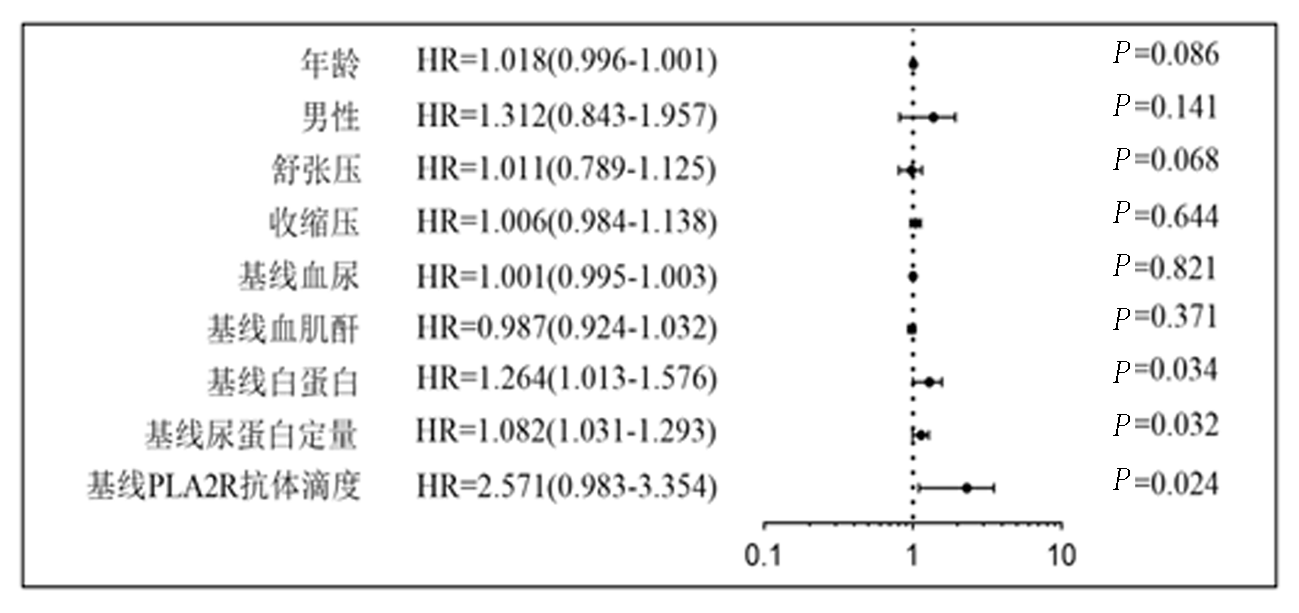
| 组别 | 例数 | 年龄 (岁) | 男 [例(%)] | 治疗基线PLA2R抗体 视屏(RU/ml) | 治疗基线Scr (μmol/L) | 治疗基线ALB (g/L) | 治疗基线 24 hUTP(g/d) | 治疗基线血尿 (个/μl) |
|---|---|---|---|---|---|---|---|---|
| IMN未缓解亚组 | 17 | 53.00±19.00 | 15(88.24) | 171.16±66.33 | 84.65±22.33 | 17.95±3.11 | 11.12±4.41 | 16.19±4.73 |
| IMN缓解亚组 | 45 | 50.00±23.00 | 40(88.89) | 90.97±21.96 | 89.18±29.35 | 19.88±5.49 | 8.73±3.31 | 19.26±5.87 |
| t/χ2值 | 0.363 | 0.019 | 3.629 | 0.388 | 1.371 | 1.935 | 1.814 | |
| P值 | 0.721 | 0.891 | 0.019 | 0.702 | 0.179 | 0.061 | 0.078 |
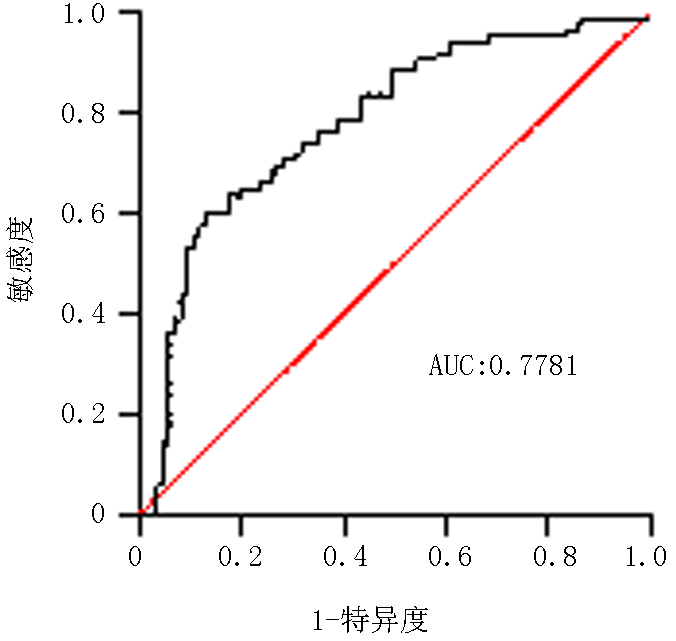
Tab. 5 Comparison of various indicators between IMN non-remission and remission subgroups in PLA2R-Ab positive group at 12 months of treatment( x -±s)
| 组别 | 例数 | 年龄 (岁) | 男 [例(%)] | 治疗基线PLA2R抗体 视屏(RU/ml) | 治疗基线Scr (μmol/L) | 治疗基线ALB (g/L) | 治疗基线 24 hUTP(g/d) | 治疗基线血尿 (个/μl) |
|---|---|---|---|---|---|---|---|---|
| IMN未缓解亚组 | 17 | 53.00±19.00 | 15(88.24) | 171.16±66.33 | 84.65±22.33 | 17.95±3.11 | 11.12±4.41 | 16.19±4.73 |
| IMN缓解亚组 | 45 | 50.00±23.00 | 40(88.89) | 90.97±21.96 | 89.18±29.35 | 19.88±5.49 | 8.73±3.31 | 19.26±5.87 |
| t/χ2值 | 0.363 | 0.019 | 3.629 | 0.388 | 1.371 | 1.935 | 1.814 | |
| P值 | 0.721 | 0.891 | 0.019 | 0.702 | 0.179 | 0.061 | 0.078 |
| [1] |
Pan X, Xu J, Ren H, et al. Changing spectrum of biopsy-proven primary glomerular diseases over the past 15 years: A single-center study in China[J]. Contrib Nephrol, 2013, 181:22-30.
doi: 10.1159/000348638 pmid: 23689564 |
| [2] |
Liu J, Li X, Huang T, et al. Efficacy and safety of 12 immunosuppressive agents for idiopathic membranous nephropathy in adults: A pairwise and network meta-analysis[J]. Front Pharmacol, 2022, 13(25):917532.
doi: 10.3389/fphar.2022.917532 URL |
| [3] |
陈瑞颖, 鲁鉴达, 谢琼虹, 等. 成人磷脂酶A2受体相关特发性膜性肾病的自然病程和治疗反应的影响因素[J]. 中华肾脏病杂志, 2019, 35(1):1-8.
doi: 10.3760/cma.j.issn.1001-7097.2019.01.001 |
| [4] |
李幼奇, 刘珍珍, 林克宣, 等. 肾组织中磷脂酶A2受体抗原的表达与特发性膜性肾病的临床及预后的关系[J]. 中华肾脏病杂志, 2018, 34(9):661-666.
doi: 10.3760/cma.j.issn.1001-7097.2018.09.004 |
| [5] |
温丽颖, 李绍梅, 闫喆, 等. M型磷脂酶A2受体及1型血小板反应蛋白7A域在成人特发性膜性肾病的表达及其意义[J]. 中华肾脏病杂志, 2016, 32(8):561-567.
doi: 10.3760/cma.j.issn.1001-7097.2016.08.001 |
| [6] |
Kidney Disease: Improving Global Outcomes KDIGO Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease[J]. Kidney Int, 2020, 98(4S):S1-S115.
doi: 10.1016/j.kint.2020.06.019 pmid: 32998798 |
| [7] |
Alsharhan L, Beck LH Jr. Membranous nephropathy:Core curriculum 2021[J]. Am J Kidney Dis, 2021, 77(3):440-453.
doi: 10.1053/j.ajkd.2020.10.009 pmid: 33487481 |
| [8] | 王述莲, 孙钧, 郑继伟, 等. 抗磷脂酶A2受体抗体表达在特发性膜性肾病人肾组织及血液中的检测价值[J]. 实用医学杂志, 2016, 32(3):434-436. |
| [9] |
Francis JM, Beck LH Jr, Salant DJ. Membranous nephropathy:A journey from bench to bedside[J]. Am J Kidney Dis, 2016, 68(1):138-147.
doi: 10.1053/j.ajkd.2016.01.030 pmid: 27085376 |
| [10] |
Luo J, Yuan Y, Tian J, et al. Clinicopathological characteristics and outcomes of PLA2R-associated membranous nephropathy in seropositive patients without PLA2R staining on kidney biopsy[J]. Am J Kidney Dis, 2022, 80(3):364-372.
doi: 10.1053/j.ajkd.2022.01.426 pmid: 35288217 |
| [11] |
Kim YG, Choi YW, Kim SY, et al. Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy[J]. Am J Nephrol, 2015, 42(3):250-257.
doi: 10.1159/000440983 pmid: 26484659 |
| [12] |
Radice A, Trezzi B, Maggiore U, et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor(PLA2R)for monitoring disease activity in idiopathic membranous nephropathy(IMN)[J]. Autoimmun Rev, 2016, 15(2):146-154.
doi: 10.1016/j.autrev.2015.10.004 URL |
| [13] |
Guo W, Zhang Y, Gao C, et al. Retrospective study: Clinicopathological features and prognosis of idiopathic membranous nephropathy with seronegative anti-phospholipase A2 receptor antibody[J]. Peer J, 2020, 8:e8650.
doi: 10.7717/peerj.8650 URL |
| [14] |
Song EJ, Jeong KH, Yang YA, et al. Anti-phospholipase A2 receptor antibody as a prognostic marker in patients with primary membranous nephropathy[J]. Kidney Res Clin Pract, 2018, 37(3):248-256.
doi: 10.23876/j.krcp.2018.37.3.248 pmid: 30254849 |
| [15] |
Hoxha E, Harendza S, Pinnschmidt HO, et al. Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy[J]. Nephrol Dial Transplant, 2015, 30(11):1862-1869.
doi: 10.1093/ndt/gfv228 URL |
| [16] | 郑娜, 汪梅花, 安智. 血清抗磷脂酶A2受体抗体与膜性肾病预后关系研究[J]. 临床军医杂志, 2021, 49(8):942-944. |
| [17] | 王媛媛, 周华. 抗磷脂酶A2受体抗体与原发性膜性肾病的临床特征和预后的相关性[J]. 中国医科大学学报, 2022, 51(2):106-110. |
| [18] |
Ruggenenti P, Debiec H, Ruggiero B, et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy[J]. J Am Soc Nephrol, 2015, 26(10):2545-2558.
doi: 10.1681/ASN.2014070640 pmid: 25804280 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||