Clinical Focus ›› 2022, Vol. 37 ›› Issue (10): 889-898.doi: 10.3969/j.issn.1004-583X.2022.10.002
Previous Articles Next Articles
Sarcopenia on immune checkpoint inhibitors in solid tumor patients with sarcopenia: A meta-analysis
Ye Qian1, Ling Zhi2,3, Yin Xudong2( )
)
- 1. Yanzhou Municipal Center for Disease Control and Prevention, Yanzhou 225001, China
2. Department of Oncology, Affiliated Hospital of Yangzhou University, jiangsu, Yangzhou 225100, China
3. Medical College of Yangzhou University, jiangsu, Yangzhou 225100, China
-
Received:2021-10-18Online:2022-10-20Published:2022-11-26 -
Contact:Yin Xudong E-mail:090005@yzu.edu.cn
CLC Number:
Cite this article
Ye Qian, Ling Zhi, Yin Xudong. Sarcopenia on immune checkpoint inhibitors in solid tumor patients with sarcopenia: A meta-analysis[J]. Clinical Focus, 2022, 37(10): 889-898.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2022.10.002
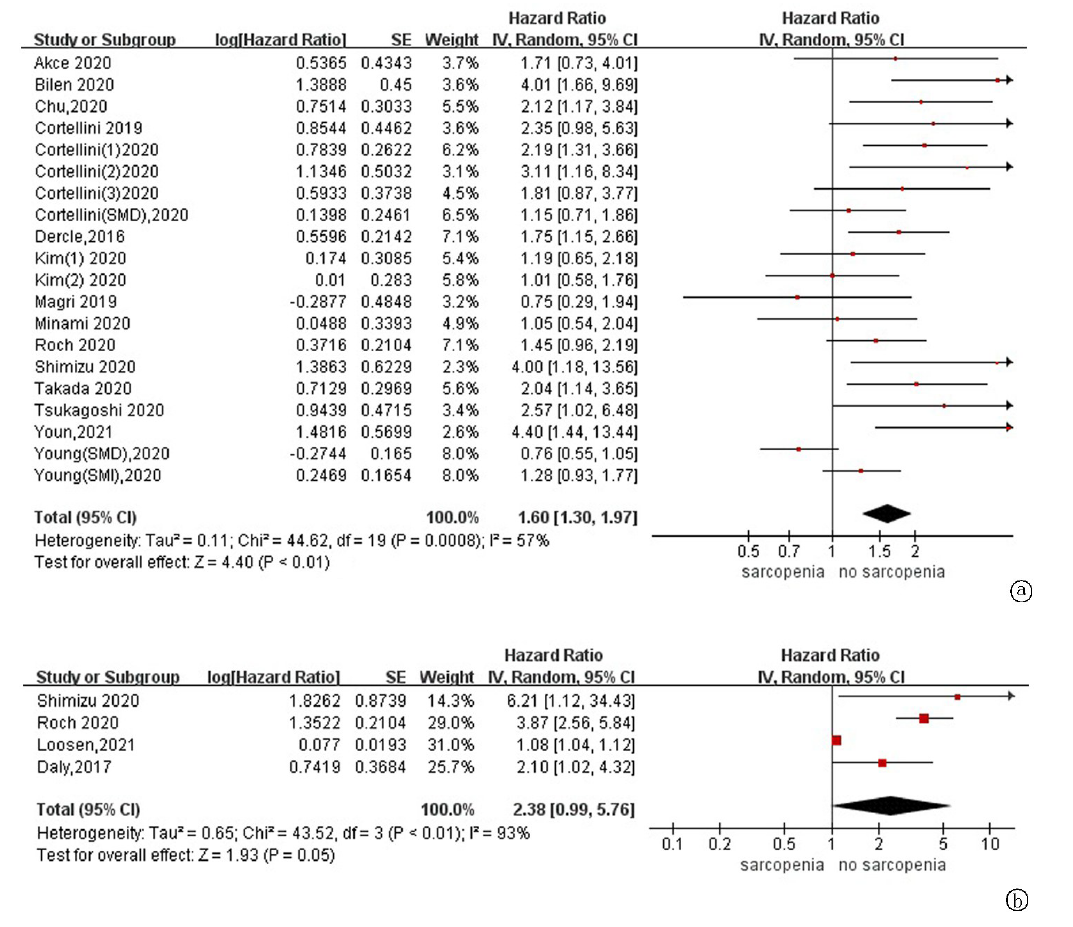
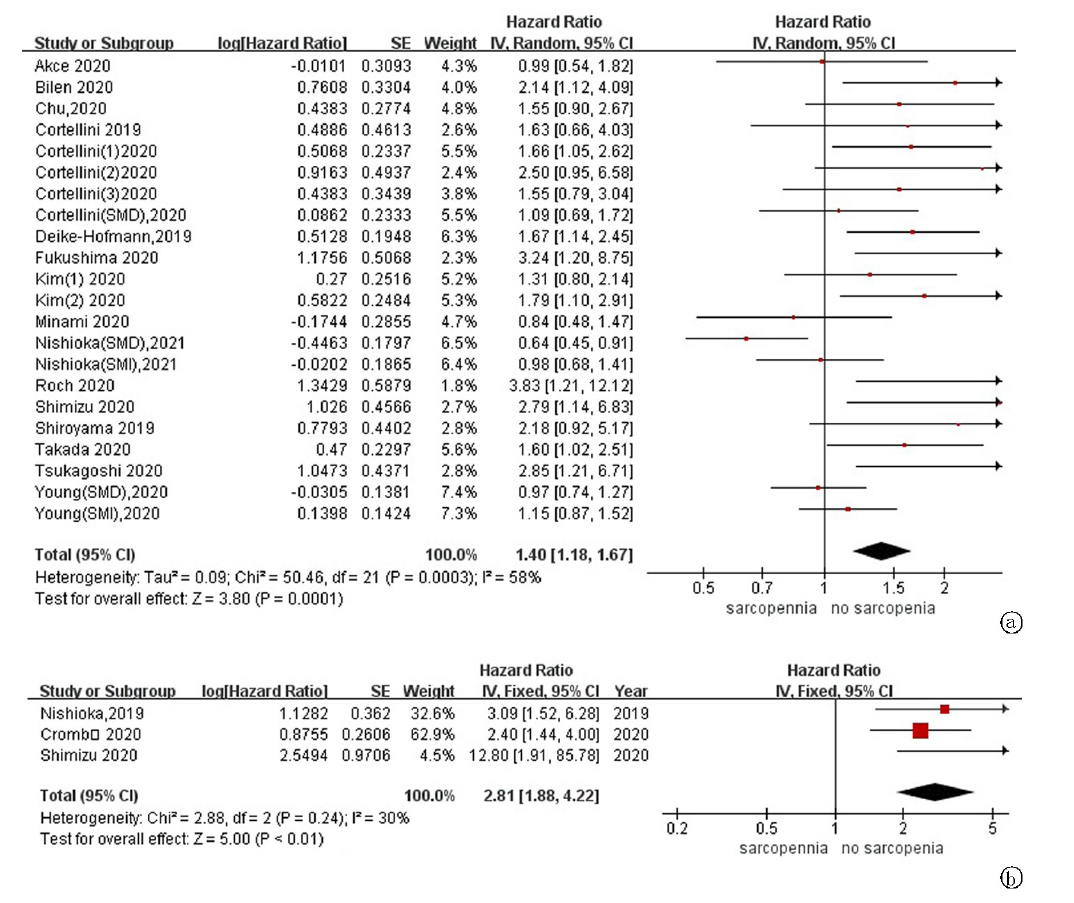
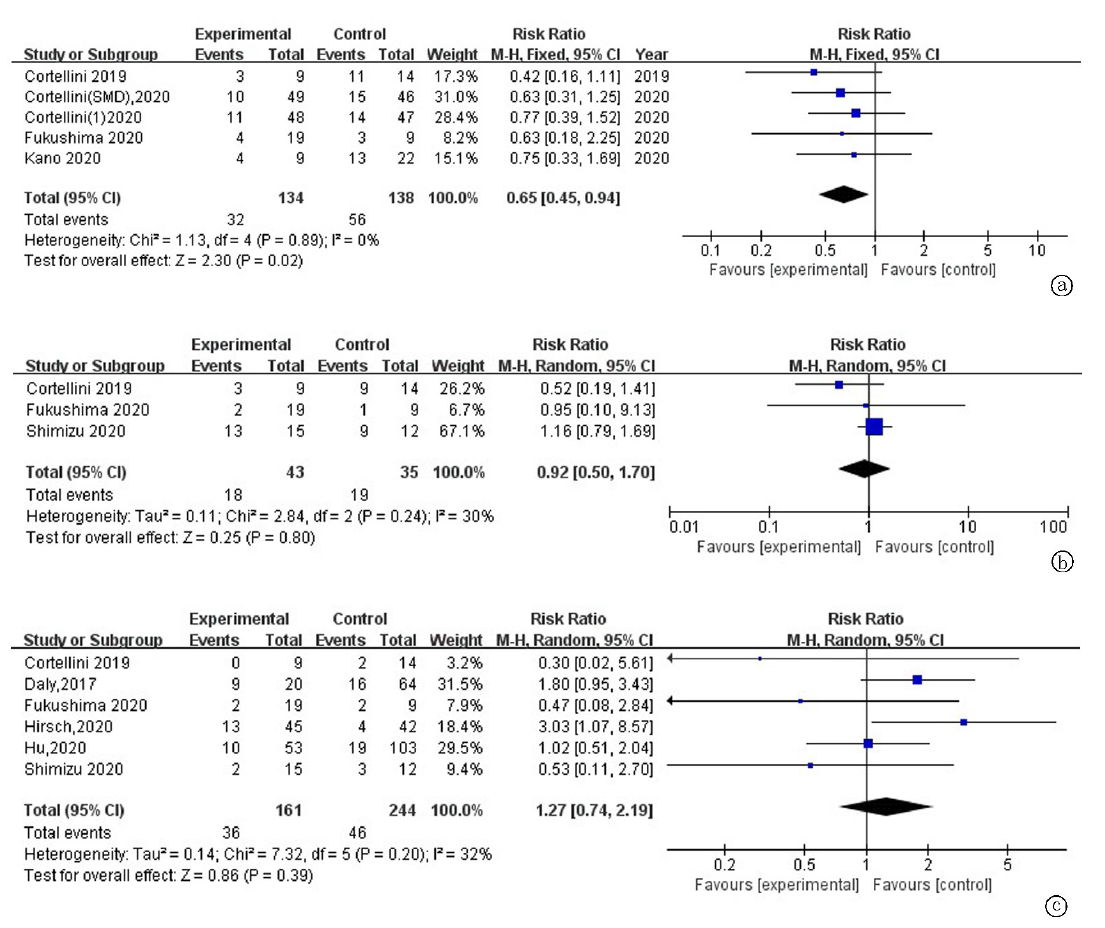
| 作者 | 发表年份 | 地区 | 样本量 | 肿瘤类型 | ICIs种类 | 诊断指标 | 测量指标 | 分界值 | 结果 | 质量评价 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dercle[ | 2016 | 法国 | 251 | 实体瘤 | PD-1/PD-L1抑制剂 | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 | OS | 高(8分) |
| Shiroyama[ | 2019 | 日本 | 42 | 肺癌 | Pembrolizumab Nivolumab | PMI by CT | 腰3层面腰大肌 | 男性:6.36 cm2/m2 女性:3.92 cm2/m2 | PFS, OS, DCR | 高(8分) |
| Nishioka[ | 2019 | 日本 | 38 | 肺癌 | Pembrolizumab Nivolumab | PMA by CT | 腰2-3层面骨骼肌 | ▲PMA≥ 10% mPMA | PFS, OS, DCR | 高(8分) |
| Cortellini[ | 2020 | 意大利 | 100 | 实体瘤 | Pembrolizumab Nivolumab Atezolizumb others | SMI by CT | 腰3层面骨骼肌 | 男性:48.4 cm2/m2 for BMI<25, 50.2 cm2/m2 for BMI≥25 女性:36.9 cm2/m2 for BMI<25, 59.6 cm2/m2 for BMI≥25 | PFS, OS, ORR, irAEs | 高(7分) |
| Crombé[ | 2020 | 法国 | 117 | 实体瘤 | PD-1/PD-L1抑制剂 | PMI by CT | 腰3层面腰大肌 | ▲PMI<三分位数最小值 | PFS | 高(7分) |
| Roch[ | 2020 | 法国 | 142 | 肺癌 | Pembrolizumab Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:52.4 cm2/m2 女性:38.5 cm2/m2 ▲SMI≥5% mSMI | PFS, OS, DCR | 高(8分) |
| Takada[ | 2020 | 日本 | 103 | 肺癌 | Pembrolizumab Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:25.63 cm2/m2 女性:21.73 cm2/m2 | PFS, OS, DCR, ORR | 高(7分) |
| Bilen[ | 2020 | 美国 | 90 | 实体瘤 | NR | SMI by CT | 腰3层面骨骼肌 | 男性:55.97 cm2/m2 女性:37.39 cm2/m2 | PFS, OS | 高(7分) |
| Kim[ | 2020 | 韩国 | 102 | 肝癌 | Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:42 cm2/m2 女性:38 cm2/m2 | PFS, OS, DCR, ORR | 高(8分) |
| Kano[ | 2020 | 日本 | 31 | 胃癌 | Nivolumab | PMI by CT | 腰3层面腰大肌 | 男性:3.6 cm2/m2 女性:2.9 cm2/m2 | PFS,DCR,ORR, irAEs | 高(7分) |
| Shimizu[ | 2020 | 日本 | 27 | 尿路上皮癌 | Pembrolizumab | PMI by CT | 腰3层面腰大肌 | 男性:6.36 cm2/m2 女性:3.92 cm2/m2 ▲PMI≥10% mPMI | PFS, OS, irAEs | 高(8分) |
| Fukushima[ | 2020 | 日本 | 28 | 尿路上皮癌 | Pembrolizumab | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 女性:25 cm2/m2 | PFS,ORR,irAEs | 高(7分) |
| Corellini[ | 2020 | 意大利 | 23 | 肺癌 | Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 | PFS,OS,ORR, irAEs | 高(7分) |
| Nishioka[ | 2021 | 日本 | 156 | 肺癌 | Pembrolizumab Nivolumab Atezolizumb | SMI和SMA by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 | PFS, ORR | 高(7分) |
| Daly[ | 2017 | 爱尔兰 | 84 | 黑色素瘤 | Ipilimumab | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI < 25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 ▲SMI≥7.5% mSMI | OS,irAEs | 中(6分) |
| Deike-Hofmann[ | 2019 | 德国 | 147 | 黑色素瘤 | Ipilimumab | PMD by CT | 腰3层面腰大肌 | 45HU(四分位数最小值) | PFS | 中(6分) |
| Chu[ | 2020 | 加拿大 | 97 | 黑色素瘤 | Ipilimumab | SMD by CT | 腰3层面骨骼肌 | 42HU for BMI<25, 20HU for BMI≥25 | PFS, OS, DCR, ORR | 中(6分) |
| Hirsch[ | 2020 | 法国 | 92 | 实体瘤 | Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 | irAEs | 中(6分) |
| Hu[ | 2020 | 美国 | 156 | 黑色素瘤 | Pembrolizumab | PMI by CT | 腰3层面腰大肌 | 男性:5.45 cm2/m2 女性:3.85 cm2/m2 | ORR, irAEs | 中(6分) |
| Young[ | 2020 | 美国 | 287 | 黑色素瘤 | Ipilimumab+Nivolumab Pembrolizumab Nivolumab Atezolizumb | SMI和SMD by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25 kg/m2, 53 cm2/m2 for BMI ≥ 25 kg/m2 女性: 41 cm2/m2 41HU for BMI<25 kg/m2, 33HU for BMI≥25 kg/m2 | PFS, OS | 中(6分) |
| Akce[ | 2020 | 美国 | 57 | 肝癌 | PD-1抑制剂 | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 女性:39 cm2/m2 | PFS, OS | 中(6分) |
| Minami[ | 2020 | 日本 | 74 | 肺癌 | Pembrolizumab Nivolumab Atezolizumb | PMI by CT | 腰3层面腰大肌 | 男性:6.36 cm2/m2 女性:3.92 cm2/m2 | PFS, OS, DCR, ORR | 中(6分) |
| Kim[ | 2020 | 韩国 | 149 | 胃癌 | Pembrolizumab Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:49 cm2/m2 女性:31 cm2/m2 | PFS, OS, DCR, ORR | 中(6分) |
| Tsukgaoshi[ | 2020 | 日本 | 30 | 肺癌 | Nivolumab | PMI by CT | 腰3层面腰大肌 | 男性:6.36 cm2/m2 女性:3.92 cm2/m2 | ORR | 中(6分) |
| Youn[ | 2021 | 加拿大 | 44 | 黑色素瘤 | Ipilimumab+Nivolumab Nivolumab | SMD by CT | 腰3层面骨骼肌 | 25.65HU | OS | 中(6分) |
| Loosen[ | 2021 | 德国 | 88 | 实体瘤 | PD-1/PD-L1抑制剂 | SMI by CT | 腰3层面骨骼肌 | ▲SMI≥6.18 mm2/cm | OS | 中(6分) |
| Magri[ | 2019 | 意大利 | 46 | 肺癌 | Nivolumab | SMI by CT | 腰3层面骨骼肌 | NR | OS | 低(4分) |
| 作者 | 发表年份 | 地区 | 样本量 | 肿瘤类型 | ICIs种类 | 诊断指标 | 测量指标 | 分界值 | 结果 | 质量评价 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dercle[ | 2016 | 法国 | 251 | 实体瘤 | PD-1/PD-L1抑制剂 | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 | OS | 高(8分) |
| Shiroyama[ | 2019 | 日本 | 42 | 肺癌 | Pembrolizumab Nivolumab | PMI by CT | 腰3层面腰大肌 | 男性:6.36 cm2/m2 女性:3.92 cm2/m2 | PFS, OS, DCR | 高(8分) |
| Nishioka[ | 2019 | 日本 | 38 | 肺癌 | Pembrolizumab Nivolumab | PMA by CT | 腰2-3层面骨骼肌 | ▲PMA≥ 10% mPMA | PFS, OS, DCR | 高(8分) |
| Cortellini[ | 2020 | 意大利 | 100 | 实体瘤 | Pembrolizumab Nivolumab Atezolizumb others | SMI by CT | 腰3层面骨骼肌 | 男性:48.4 cm2/m2 for BMI<25, 50.2 cm2/m2 for BMI≥25 女性:36.9 cm2/m2 for BMI<25, 59.6 cm2/m2 for BMI≥25 | PFS, OS, ORR, irAEs | 高(7分) |
| Crombé[ | 2020 | 法国 | 117 | 实体瘤 | PD-1/PD-L1抑制剂 | PMI by CT | 腰3层面腰大肌 | ▲PMI<三分位数最小值 | PFS | 高(7分) |
| Roch[ | 2020 | 法国 | 142 | 肺癌 | Pembrolizumab Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:52.4 cm2/m2 女性:38.5 cm2/m2 ▲SMI≥5% mSMI | PFS, OS, DCR | 高(8分) |
| Takada[ | 2020 | 日本 | 103 | 肺癌 | Pembrolizumab Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:25.63 cm2/m2 女性:21.73 cm2/m2 | PFS, OS, DCR, ORR | 高(7分) |
| Bilen[ | 2020 | 美国 | 90 | 实体瘤 | NR | SMI by CT | 腰3层面骨骼肌 | 男性:55.97 cm2/m2 女性:37.39 cm2/m2 | PFS, OS | 高(7分) |
| Kim[ | 2020 | 韩国 | 102 | 肝癌 | Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:42 cm2/m2 女性:38 cm2/m2 | PFS, OS, DCR, ORR | 高(8分) |
| Kano[ | 2020 | 日本 | 31 | 胃癌 | Nivolumab | PMI by CT | 腰3层面腰大肌 | 男性:3.6 cm2/m2 女性:2.9 cm2/m2 | PFS,DCR,ORR, irAEs | 高(7分) |
| Shimizu[ | 2020 | 日本 | 27 | 尿路上皮癌 | Pembrolizumab | PMI by CT | 腰3层面腰大肌 | 男性:6.36 cm2/m2 女性:3.92 cm2/m2 ▲PMI≥10% mPMI | PFS, OS, irAEs | 高(8分) |
| Fukushima[ | 2020 | 日本 | 28 | 尿路上皮癌 | Pembrolizumab | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 女性:25 cm2/m2 | PFS,ORR,irAEs | 高(7分) |
| Corellini[ | 2020 | 意大利 | 23 | 肺癌 | Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 | PFS,OS,ORR, irAEs | 高(7分) |
| Nishioka[ | 2021 | 日本 | 156 | 肺癌 | Pembrolizumab Nivolumab Atezolizumb | SMI和SMA by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 | PFS, ORR | 高(7分) |
| Daly[ | 2017 | 爱尔兰 | 84 | 黑色素瘤 | Ipilimumab | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI < 25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 ▲SMI≥7.5% mSMI | OS,irAEs | 中(6分) |
| Deike-Hofmann[ | 2019 | 德国 | 147 | 黑色素瘤 | Ipilimumab | PMD by CT | 腰3层面腰大肌 | 45HU(四分位数最小值) | PFS | 中(6分) |
| Chu[ | 2020 | 加拿大 | 97 | 黑色素瘤 | Ipilimumab | SMD by CT | 腰3层面骨骼肌 | 42HU for BMI<25, 20HU for BMI≥25 | PFS, OS, DCR, ORR | 中(6分) |
| Hirsch[ | 2020 | 法国 | 92 | 实体瘤 | Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25, 53 cm2/m2 for BMI≥25 女性: 41 cm2/m2 | irAEs | 中(6分) |
| Hu[ | 2020 | 美国 | 156 | 黑色素瘤 | Pembrolizumab | PMI by CT | 腰3层面腰大肌 | 男性:5.45 cm2/m2 女性:3.85 cm2/m2 | ORR, irAEs | 中(6分) |
| Young[ | 2020 | 美国 | 287 | 黑色素瘤 | Ipilimumab+Nivolumab Pembrolizumab Nivolumab Atezolizumb | SMI和SMD by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 for BMI<25 kg/m2, 53 cm2/m2 for BMI ≥ 25 kg/m2 女性: 41 cm2/m2 41HU for BMI<25 kg/m2, 33HU for BMI≥25 kg/m2 | PFS, OS | 中(6分) |
| Akce[ | 2020 | 美国 | 57 | 肝癌 | PD-1抑制剂 | SMI by CT | 腰3层面骨骼肌 | 男性:43 cm2/m2 女性:39 cm2/m2 | PFS, OS | 中(6分) |
| Minami[ | 2020 | 日本 | 74 | 肺癌 | Pembrolizumab Nivolumab Atezolizumb | PMI by CT | 腰3层面腰大肌 | 男性:6.36 cm2/m2 女性:3.92 cm2/m2 | PFS, OS, DCR, ORR | 中(6分) |
| Kim[ | 2020 | 韩国 | 149 | 胃癌 | Pembrolizumab Nivolumab | SMI by CT | 腰3层面骨骼肌 | 男性:49 cm2/m2 女性:31 cm2/m2 | PFS, OS, DCR, ORR | 中(6分) |
| Tsukgaoshi[ | 2020 | 日本 | 30 | 肺癌 | Nivolumab | PMI by CT | 腰3层面腰大肌 | 男性:6.36 cm2/m2 女性:3.92 cm2/m2 | ORR | 中(6分) |
| Youn[ | 2021 | 加拿大 | 44 | 黑色素瘤 | Ipilimumab+Nivolumab Nivolumab | SMD by CT | 腰3层面骨骼肌 | 25.65HU | OS | 中(6分) |
| Loosen[ | 2021 | 德国 | 88 | 实体瘤 | PD-1/PD-L1抑制剂 | SMI by CT | 腰3层面骨骼肌 | ▲SMI≥6.18 mm2/cm | OS | 中(6分) |
| Magri[ | 2019 | 意大利 | 46 | 肺癌 | Nivolumab | SMI by CT | 腰3层面骨骼肌 | NR | OS | 低(4分) |
| 组别 | HR(95%CI) | P值 |
|---|---|---|
| 肿瘤类型 | ||
| 肺癌 | 1.57(1.22~2.0) | 0.000 |
| 黑色素瘤 | 1.41(0.84~2.38) | 0.190 |
| 其他 | 1.73(1.29~2.32) | 0.000 |
| ICIs类型 | ||
| Pembrolizumab | 4.0(1.18~13.56) | 0.030 |
| nivolumab | 1.49(0.88~2.52) | 0.013 |
| Ipilimumab | 2.12(1.17~3.84) | 0.010 |
| 其他 | 1.55(1.22~1.97) | 0.000 |
| 诊断指标 | ||
| SMI | 1.62(1.33~1.97) | 0.000 |
| PMI | 1.97(0.88~4.42) | 0.100 |
| SMD | 1.47(0.79~2.75) | 0.230 |
| PMD | - | - |
| 地区 | ||
| 亚洲 | 1.41(0.98~2.01) | 0.060 |
| 欧美 | 1.71(1.31~2.22) | 0.000 |
| 组别 | HR(95%CI) | P值 |
|---|---|---|
| 肿瘤类型 | ||
| 肺癌 | 1.57(1.22~2.0) | 0.000 |
| 黑色素瘤 | 1.41(0.84~2.38) | 0.190 |
| 其他 | 1.73(1.29~2.32) | 0.000 |
| ICIs类型 | ||
| Pembrolizumab | 4.0(1.18~13.56) | 0.030 |
| nivolumab | 1.49(0.88~2.52) | 0.013 |
| Ipilimumab | 2.12(1.17~3.84) | 0.010 |
| 其他 | 1.55(1.22~1.97) | 0.000 |
| 诊断指标 | ||
| SMI | 1.62(1.33~1.97) | 0.000 |
| PMI | 1.97(0.88~4.42) | 0.100 |
| SMD | 1.47(0.79~2.75) | 0.230 |
| PMD | - | - |
| 地区 | ||
| 亚洲 | 1.41(0.98~2.01) | 0.060 |
| 欧美 | 1.71(1.31~2.22) | 0.000 |
| 组别 | HR(95%CI) | P值 |
|---|---|---|
| 肿瘤类型 | ||
| 肺癌 | 1.36(0.95~1.95) | 0.090 |
| 黑色素瘤 | 1.24(0.97~1.59) | 0.090 |
| 其他 | 1.6(1.26~2.02) | <0.01 |
| ICIs类型 | ||
| Pembrolizumab | 2.98(1.53~5.80) | 0.001 |
| Nivolumab | 1.64(1.06~2.55) | 0.030 |
| Ipilimumab | 1.63(1.19~2.23) | 0.002 |
| 其他 | 1.26(1.04~1.54) | 0.020 |
| 诊断指标 | ||
| SMI | 1.47(1.22~1.77) | <0.01 |
| PMI | 1.87(0.94~3.59) | 0.070 |
| SMD | 0.97(0.70~1.34) | 0.860 |
| PMD | 1.67(1.14~2.45) | 0.008 |
| 地区 | ||
| 亚洲 | 1.44(1.03~2.01) | 0.030 |
| 欧美 | 1.38(1.15~1.66) | 0.000 |
| 组别 | HR(95%CI) | P值 |
|---|---|---|
| 肿瘤类型 | ||
| 肺癌 | 1.36(0.95~1.95) | 0.090 |
| 黑色素瘤 | 1.24(0.97~1.59) | 0.090 |
| 其他 | 1.6(1.26~2.02) | <0.01 |
| ICIs类型 | ||
| Pembrolizumab | 2.98(1.53~5.80) | 0.001 |
| Nivolumab | 1.64(1.06~2.55) | 0.030 |
| Ipilimumab | 1.63(1.19~2.23) | 0.002 |
| 其他 | 1.26(1.04~1.54) | 0.020 |
| 诊断指标 | ||
| SMI | 1.47(1.22~1.77) | <0.01 |
| PMI | 1.87(0.94~3.59) | 0.070 |
| SMD | 0.97(0.70~1.34) | 0.860 |
| PMD | 1.67(1.14~2.45) | 0.008 |
| 地区 | ||
| 亚洲 | 1.44(1.03~2.01) | 0.030 |
| 欧美 | 1.38(1.15~1.66) | 0.000 |
| [1] |
Floudas CS, Brar G, Greten TF. Immunotherapy: Current status and future perspectives[J]. Dig Dis Sci, 2019, 64(4):1030-1040.
doi: 10.1007/s10620-019-05516-7 URL |
| [2] |
Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors[J]. Cancer Cell, 2020, 37(4):443-455.
doi: S1535-6108(20)30157-4 pmid: 32289269 |
| [3] |
Bai R, Lv Z, Xu D, et al. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors[J]. Biomark Res, 2020, 8:34.
doi: 10.1186/s40364-020-00209-0 pmid: 32864131 |
| [4] |
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis[J]. Age Ageing, 2019, 48(4):601.
doi: 10.1093/ageing/afz046 pmid: 31081853 |
| [5] |
Morosano ME, Menoyo IM, Tomat MF, et al. A simple anthropometric tool for the assessment of pre-sarcopenia in postmenopausal women[J]. Climacteric, 2017, 20(3):256-261.
doi: 10.1080/13697137.2017.1309017 pmid: 28379719 |
| [6] |
Ofek Shlomai N, Rao S, Patole S. Efficacy of interventions to improve hand hygiene compliance in neonatal units: A systematic review and meta-analysis[J]. Eur J Clin Microbiol Infect Dis, 2015, 34(5):887-897.
doi: 10.1007/s10096-015-2313-1 URL |
| [7] |
Dercle L, Ammari S, Champiat S, et al. Rapid and objective CT scan prognostic scoring identifies Metastatic patients with long-term clinical benefit on anti-PD-1/-L1 therapy[J]. Eur J Cancer, 2016, 65:33-42.
doi: 10.1016/j.ejca.2016.05.031 URL |
| [8] |
Shiroyama T, Nagatomo I, Koyama S, et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study[J]. Sci Rep, 2019, 9(1):2447.
doi: 10.1038/s41598-019-39120-6 pmid: 30792455 |
| [9] |
Nishioka N, Uchino J, Hirai S, et al. Association of sarcopenia with and efficacy of anti-PD-1/PD-L1 therapy in non-small-cell lung cancer[J]. J Clin Med, 2019, 8(4):450.
doi: 10.3390/jcm8040450 URL |
| [10] |
Cortellini A, Bozzetti F, Palumbo P, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: A multicenter real-life study[J]. Sci Rep, 2020, 10(1):1456.
doi: 10.1038/s41598-020-58498-2 pmid: 31996766 |
| [11] |
Crombé A, Kind M, Toulmonde M, et al. Impact of CT-based body composition parameters at baseline, their early changes and response in metastatic cancer patients treated with immune checkpoint inhibitors[J]. Eur J Radiol, 2020, 133:109340.
doi: 10.1016/j.ejrad.2020.109340 URL |
| [12] |
Roch B, Coffy A, Jean-Baptiste S, et al. Cachexia-sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors[J]. Lung Cancer, 2020, 143:19-26.
doi: 10.1016/j.lungcan.2020.03.003 URL |
| [13] |
Takada K, Yoneshima Y, Tanaka K, et al. Clinical impact of skeletal muscle area in patients with non-small cell lung cancer treated with anti-PD-1 inhibitors[J]. J Cancer Res Clin Oncol, 2020, 146(5):1217-1225.
doi: 10.1007/s00432-020-03146-5 pmid: 32025867 |
| [14] |
Bilen MA, Martini DJ, Liu Y, et al. Combined effect of sarcopenia and systemic inflammation on survival in patients with advanced stage cancer treated with immunotherapy[J]. Oncologist, 2020, 25(3):e528-e535.
doi: 10.1634/theoncologist.2019-0751 URL |
| [15] |
Kim N, Yu JI, Park HC, et al. Incorporating sarcopenia and inflammation with radiation therapy in patients with hepatocellular carcinoma treated with nivolumab[J]. Cancer Immunol Immunother, 2021, 70(6):1593-1603.
doi: 10.1007/s00262-020-02794-3 pmid: 33231725 |
| [16] |
Kano M, Hihara J, Tokumoto N, et al. Association between skeletal muscle loss and the response to nivolumab immunotherapy in advanced gastric cancer patients[J]. Int J Clin Oncol 2021, 26(3):523-531.
doi: 10.1007/s10147-020-01833-4 pmid: 33226523 |
| [17] | Shimizu T, Miyake M, Hori S, et al. Clinical impact of sarcopenia and inflammatory/nutritional markers in patients with unresectable Metastatic urothelial carcinoma treated with pembrolizumab[J]. Diagnostics (Basel), 2020, 10(5). |
| [18] |
Fukushima H, Fukuda S, Moriyama S, et al. Impact of sarcopenia on the efficacy of pembrolizumab in patients with advanced urothelial carcinoma: A preliminary report[J]. Anticancer Drugs, 2020, 31(8):866-871.
doi: 10.1097/CAD.0000000000000982 pmid: 32740015 |
| [19] |
Cortellini A, Verna L, Porzio G, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: A "hypothesis-generator" preliminary report[J]. Thorac Cancer, 2019, 10(2):347-351.
doi: 10.1111/1759-7714.12965 pmid: 30600905 |
| [20] |
Nishioka N, Naito T, Notsu A, et al. Unfavorable impact of decreased muscle quality on the efficacy of immunotherapy for advanced non-small cell lung cancer[J]. Cancer Med, 2021, 10(1):247-256.
doi: 10.1002/cam4.3631 URL |
| [21] |
Daly LE, Power DG, O'Reilly A, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with Metastatic melanoma[J]. Br J Cancer 2017, 116(3):310-317.
doi: 10.1038/bjc.2016.431 URL |
| [22] |
Deike-Hofmann K, Gutzweiler L, Reuter J, et al. Macroangiopathy is a positive predictive factor for response to immunotherapy[J]. Sci Rep, 2019, 9(1):9728.
doi: 10.1038/s41598-019-46189-6 pmid: 31278360 |
| [23] |
Chu MP, Li Y, Ghosh S, Sass S, et al. Body composition is prognostic and predictive of ipilimumab activity in Metastatic melanoma[J]. J Cachexia Sarcopenia Muscle, 2020, 11(3):748-755.
doi: 10.1002/jcsm.12538 pmid: 32053287 |
| [24] |
Hirsch L, Bellesoeur A, Boudou-Rouquette P, et al. The impact of body composition parameters on severe toxicity of nivolumab[J]. Eur J Cancer, 2020, 124:170-177.
doi: S0959-8049(19)30810-X pmid: 31794927 |
| [25] |
Hu JB, Ravichandran S, Rushing C, et al. Higher BMI, but not sarcopenia, is associated with pembrolizumab-related toxicity in patients with advanced melanoma[J]. Anticancer Res, 2020, 40(9):5245-5254.
doi: 10.21873/anticanres.14528 pmid: 32878813 |
| [26] | Young AC, Quach HT, Song H, et al. Impact of body composition on outcomes from anti-PD1 +/- anti-CTLA-4 treatment in melanoma[J]. J Immunother Cancer, 2020, 8(2). |
| [27] |
Akce M, Liu Y, Zakka K, Martini DJ, et al. Impact of sarcopenia, BMI, and inflammatory biomarkers on survival in advanced hepatocellular carcinoma treated with anti-PD-1 antibody[J]. Am J Clin Oncol, 2021, 44(2):74-81.
doi: 10.1097/COC.0000000000000787 URL |
| [28] |
Minami S, Ihara S, Tanaka T, et al. Sarcopenia and visceral adiposity did not affect efficacy of immune-checkpoint inhibitor monotherapy for pretreated patients with advanced non-small cell lung cancer[J]. World J Oncol, 2020, 11(1):9-22.
doi: 10.14740/wjon1225 pmid: 32095185 |
| [29] |
Kim YY, Lee J, Jeong WK, et al. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors[J]. Gastric Cancer, 2021, 24(2):457-466.
doi: 10.1007/s10120-020-01124-x URL |
| [30] |
Tsukagoshi M, Yokobori T, Yajima T, et al. Skeletal muscle mass predicts the outcome of nivolumab treatment for non-small cell lung cancer[J]. Medicine (Baltimore), 2020, 99(7):e19059.
doi: 10.1097/MD.0000000000019059 URL |
| [31] |
Youn S, Reif R, Chu MP, et al. Myosteatosis is prognostic in Metastatic melanoma treated with nivolumab[J]. Clin Nutr ESPEN, 2021, 42:348-353.
doi: 10.1016/j.clnesp.2021.01.009 pmid: 33745604 |
| [32] | Loosen SH, van den Bosch V, et al. Progressive sarcopenia correlates with poor response and outcome to immune checkpoint inhibitor therapy[J]. J Clin Med, 2021, 10(7). |
| [33] |
Magri V, Gottfried T, Di Segni M, et al. Correlation of body composition by computerized tomography and Metabolic parameters with survival of nivolumab-treated lung cancer patients[J]. Cancer Manag Res, 2019, 11:8201-8207.
doi: 10.2147/CMAR.S210958 pmid: 31564979 |
| [34] |
Deng HY, Chen ZJ, Qiu XM, et al. Sarcopenia and prognosis of advanced cancer patients receiving immune checkpoint inhibitors: A comprehensive systematic review and Meta-analysis[J]. Nutrition, 2021, 90:111345.
doi: 10.1016/j.nut.2021.111345 URL |
| [35] |
Wang J, Cao L, Xu S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: A systematic review and meta-analysis[J]. Int Immunopharmacol, 2020, 88:106907.
doi: 10.1016/j.intimp.2020.106907 URL |
| [36] | Quinn LS. Interleukin-15: A muscle-derived cytokine regulating fat-to-lean body composition[J]. J Anim Sci, 2008, 86(14 Suppl):E75-83. |
| [37] | Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: Altered cytokine levels as a common mechanism[J]. Aging (Albany NY), 2012, 4(8):535-546. |
| [38] |
Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of il6 and pd-1/pd-l1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment[J]. Cancer Res, 2018, 78(17):5011-5022.
doi: 10.1158/0008-5472.CAN-18-0118 pmid: 29967259 |
| [39] |
Banna GL, Di Quattro R, Malatino L, et al. Neutrophil-to-lymphocyte ratio and lactate dehydrogenase as biomarkers for urothelial cancer treated with immunotherapy[J]. Clin Transl Oncol, 2020, 22(11):2130-2135.
doi: 10.1007/s12094-020-02337-3 URL |
| [40] |
Indrakusuma R, Drudi LM. Psoas muscle area and sarcopenia-bridging the gap[J]. Eur J Vasc Endovasc Surg, 2019, 58(2):199.
doi: 10.1016/j.ejvs.2019.03.032 URL |
| [41] |
Baracos VE. Psoas as a sentinel muscle for sarcopenia: A flawed premise[J]. J Cachexia Sarcopenia Muscle, 2017, 8(4):527-528.
doi: 10.1002/jcsm.12221 pmid: 28675689 |
| [42] |
Rutten IJG, Ubachs J, Kruitwagen R, et al. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer[J]. J Cachexia Sarcopenia Muscle, 2017, 8(4):630-638.
doi: 10.1002/jcsm.12180 pmid: 28513088 |
| [43] | Anjanappa M, Corden M, Green A, et al. Sarcopenia in cancer: Risking more than muscle loss[J]. Tech Innov Patient Support Radiat Oncol, 2020, 16:50-57. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 67
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 304
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||