

临床荟萃 ›› 2023, Vol. 38 ›› Issue (6): 493-499.doi: 10.3969/j.issn.1004-583X.2023.06.002
收稿日期:2022-08-23
出版日期:2023-06-20
发布日期:2023-08-18
通讯作者:
倪艺芸,Email:
Ni Yiyun1( ), Liu Bin1, Liang Qi2, Li Xiaofeng3
), Liu Bin1, Liang Qi2, Li Xiaofeng3
Received:2022-08-23
Online:2023-06-20
Published:2023-08-18
Contact:
Ni Yiyun, Email: 摘要:
目的 探讨白介素-6(IL-6)和C反应蛋白(CRP)预测新型冠状病毒肺炎(COVID-19)严重程度的临床价值。方法 计算机检索PubMed、Web of Science、Scopus、Embase、中国知网(CNKI)、万方数据知识服务平台(Wanfang Data)、维普数据库(VIP)及中国生物医学文献数据库(CBM),搜集以COVID-19为主题的相关研究,检索时间为2019-12-01至2021-01-16,由两名研究人员独立筛选文献、提取资料,采用RevMan5.4软件进行meta分析。结果 共纳入18篇文献,共计4286例COVID-19患者,其中轻症组2662例,重症组1624例。与轻症组比较,重症组IL-6水平显著高于轻症组,差异有统计学意义( SMD =0.92,95% CI :0.65~1.18, P <0.01),重症组CRP水平也显著高于轻症组,差异有统计学意义( SMD =1.47,95% CI :1.03~1.91, P <0.01)。结论 IL-6和CRP水平的升高与COVID-19的严重程度呈正相关,可作为临床COVID-19患者风险分层的炎症指标,为临床治疗提供有意义的依据。
中图分类号:
倪艺芸, 刘彬, 梁琪, 李晓凤. 白细胞介素6和C反应蛋白预测新型冠状病毒肺炎严重程度的meta分析[J]. 临床荟萃, 2023, 38(6): 493-499.
Ni Yiyun, Liu Bin, Liang Qi, Li Xiaofeng. Values of IL-6 and CRP in predicting the severity of coronavirus disease 2019: A meta-analysis[J]. Clinical Focus, 2023, 38(6): 493-499.
| 纳入研究 | 研究类型 | 研究来源 | 总样本量 | 性别 (男/女) | 平均数/中位数 年龄(岁) | NOS评分 |
|---|---|---|---|---|---|---|
| Liu 2020[ | 单中心回顾性研究 | 中国,武汉 | 294 | 162/132 | 56(39~67) | 6 |
| Jurado 2020[ | 多中心回顾性研究 | 西班牙,多个地区 | 584 | 349/235 | 63.0±16.5 | 8 |
| Yi 2020[ | 单中心回顾性研究 | 中国,浙江 | 100 | 63/37 | 54(42~64) | 6 |
| Zhang 2020[ | 单中心回顾性研究 | 中国,武汉 | 134 | 87/47 | 60.8±13.0 | 8 |
| Yuan 2020[ | 单中心回顾性研究 | 中国,福建 | 117 | 56/61 | 66(57~71) | 6 |
| Wei 2020[ | 单中心回顾性研究 | 中国,武汉 | 565 | 289/276 | 66(59~72) | 6 |
| Liao 2020[ | 多中心回顾性研究 | 中国,武汉 | 294 | 156/138 | 64(53~73) | 8 |
| Gao 2020[ | 单中心回顾性研究 | 中国,黑龙江 | 126 | 66/60 | 64.9±12.8 | 6 |
| Shi 2020[ | 多中心回顾性研究 | 中国,陕西 | 134 | 65/69 | 46(34~58) | 8 |
| Lv 2020[ | 单中心回顾性研究 | 中国,武汉 | 270 | 135/135 | 62.0±11.2 | 6 |
| Wang 2020[ | 单中心回顾性研究 | 中国,武汉 | 123 | 60/63 | 68(56~78) | 8 |
| 孙昀 2020[ | 多中心回顾性研究 | 中国,安徽 | 168 | 95/73 | 42.6±15.8 | 6 |
| 杨秀红 2020[ | 单中心回顾性研究 | 中国,武汉 | 365 | 162/203 | 60.2±14.2 | 8 |
| 魏剑浩 2020[ | 多中心回顾性研究 | 中国,上海 | 328 | 170/158 | 51.0±12.0 | 6 |
| 吕宁 2020[ | 单中心回顾性研究 | 中国,深圳 | 246 | 119/127 | 44.0±13.2 | 6 |
| 张永喜 2020[ | 单中心回顾性研究 | 中国,武汉 | 203 | 108/95 | 54.0±11.8 | 6 |
| 邹义龙 2020[ | 单中心回顾性研究 | 中国,武汉 | 83 | 36/47 | 67.0±12.9 | 6 |
| 刘浩 2020[ | 单中心回顾性研究 | 中国,武汉 | 152 | 84/68 | 56.5±13.5 | 6 |
表1 纳入文献的基本特征及质量评价
Tab. 1 Basic characteristics and quality evaluation of the eligible literature
| 纳入研究 | 研究类型 | 研究来源 | 总样本量 | 性别 (男/女) | 平均数/中位数 年龄(岁) | NOS评分 |
|---|---|---|---|---|---|---|
| Liu 2020[ | 单中心回顾性研究 | 中国,武汉 | 294 | 162/132 | 56(39~67) | 6 |
| Jurado 2020[ | 多中心回顾性研究 | 西班牙,多个地区 | 584 | 349/235 | 63.0±16.5 | 8 |
| Yi 2020[ | 单中心回顾性研究 | 中国,浙江 | 100 | 63/37 | 54(42~64) | 6 |
| Zhang 2020[ | 单中心回顾性研究 | 中国,武汉 | 134 | 87/47 | 60.8±13.0 | 8 |
| Yuan 2020[ | 单中心回顾性研究 | 中国,福建 | 117 | 56/61 | 66(57~71) | 6 |
| Wei 2020[ | 单中心回顾性研究 | 中国,武汉 | 565 | 289/276 | 66(59~72) | 6 |
| Liao 2020[ | 多中心回顾性研究 | 中国,武汉 | 294 | 156/138 | 64(53~73) | 8 |
| Gao 2020[ | 单中心回顾性研究 | 中国,黑龙江 | 126 | 66/60 | 64.9±12.8 | 6 |
| Shi 2020[ | 多中心回顾性研究 | 中国,陕西 | 134 | 65/69 | 46(34~58) | 8 |
| Lv 2020[ | 单中心回顾性研究 | 中国,武汉 | 270 | 135/135 | 62.0±11.2 | 6 |
| Wang 2020[ | 单中心回顾性研究 | 中国,武汉 | 123 | 60/63 | 68(56~78) | 8 |
| 孙昀 2020[ | 多中心回顾性研究 | 中国,安徽 | 168 | 95/73 | 42.6±15.8 | 6 |
| 杨秀红 2020[ | 单中心回顾性研究 | 中国,武汉 | 365 | 162/203 | 60.2±14.2 | 8 |
| 魏剑浩 2020[ | 多中心回顾性研究 | 中国,上海 | 328 | 170/158 | 51.0±12.0 | 6 |
| 吕宁 2020[ | 单中心回顾性研究 | 中国,深圳 | 246 | 119/127 | 44.0±13.2 | 6 |
| 张永喜 2020[ | 单中心回顾性研究 | 中国,武汉 | 203 | 108/95 | 54.0±11.8 | 6 |
| 邹义龙 2020[ | 单中心回顾性研究 | 中国,武汉 | 83 | 36/47 | 67.0±12.9 | 6 |
| 刘浩 2020[ | 单中心回顾性研究 | 中国,武汉 | 152 | 84/68 | 56.5±13.5 | 6 |
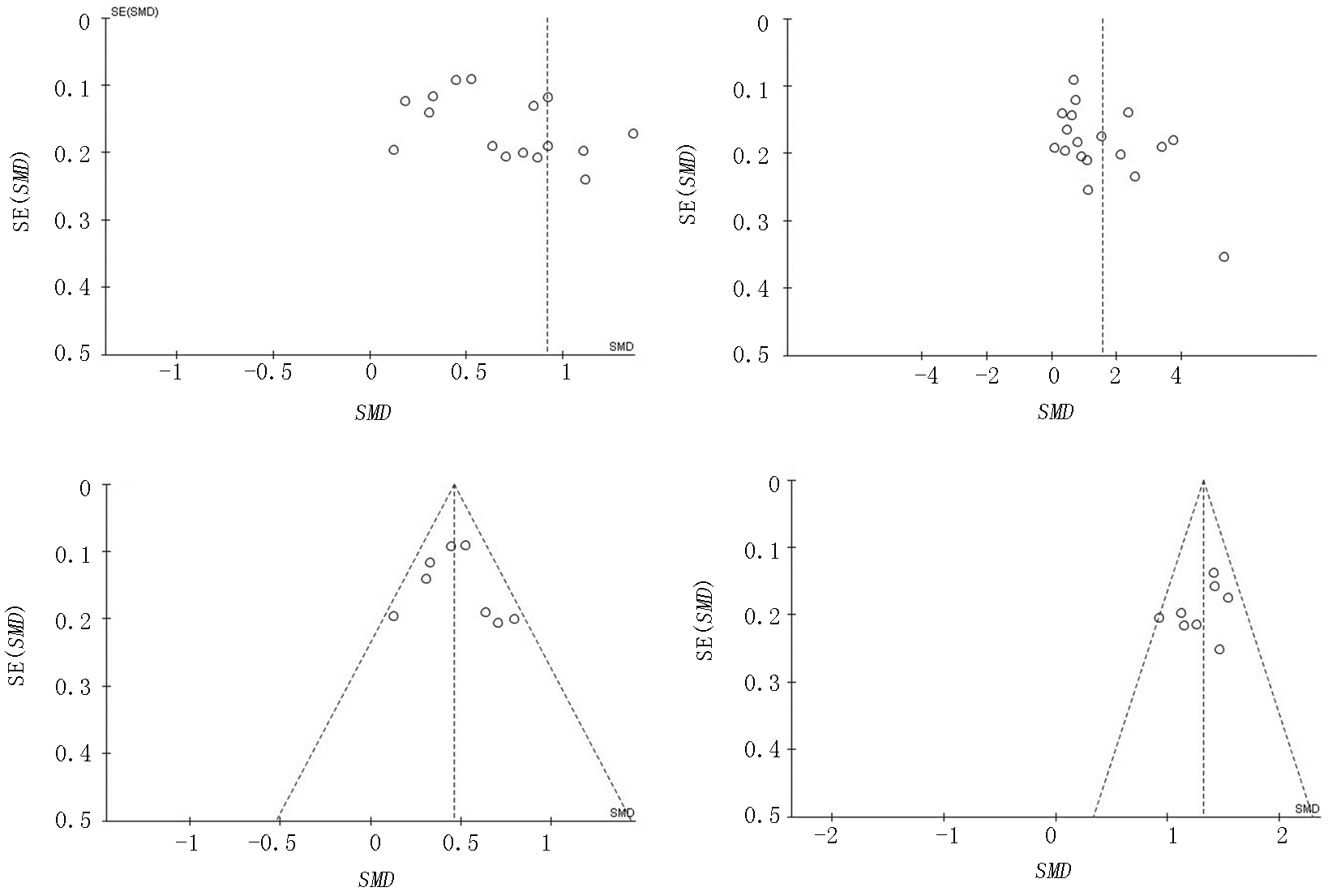
图6 重症组与轻症组IL-6和CRP水平比较漏斗图 a、b分别对应IL-6、CRP; c、d 分别剔除异质性文献后对应IL-6、CRP
Fig. 6 Funnel plots of IL-6 and CRP levels of the severe group versus the mild group a,b:diagram corresponds to IL-6 and CRP respectively; c,d:diagram corresponds to IL-6 and CRP except heterogeneous literature
| [1] | World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[R]. 2020. |
| [2] | Coronavirus COVID-19 Global Cases[EB/OL]. https://covid19.who.int/.2021-04-09. |
| [3] | World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected., Interim Guidance[EB/OL]. https://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf. 2021-04-09. |
| [4] | 中华人民共和国国家卫生健康委员会. 关于印发新型冠状病毒肺炎诊疗方案(试行第八版)[EB/OL]. http://www.gov.cn/zhengce/zhengceku/202008/19/5535757/files/da89edf7cc9244fbb34ecf6c61df40bf.pdf. |
| [5] |
Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China[J]. N Engl J Med, 2020, 382(18):1708-1720.
doi: 10.1056/NEJMoa2002032 URL |
| [6] | Liao XL, Chen H, Li Z, et al. Critical care for severe coronavirus disease 2019: a population-based study from a province with low case-fatality rate in China[J]. Chin Med J (Engl), 2020, 134(1):98-100. |
| [7] | Wu R, Ai S, Cai J, et al. Predictive model and risk factors for case fatality of COVID-19: A cohort of 21, 392 cases in Hubei, China[J]. Innovation (Camb), 2020, 1(2):100022. |
| [8] |
Moore JB, June CH. Cytokine release syndrome in severe COVID-19[J]. Science, 2020, 368(6490):473-474.
doi: 10.1126/science.abb8925 pmid: 32303591 |
| [9] |
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome[J]. Lancet Respir Med, 2020, 8(4):420-422.
doi: 10.1016/S2213-2600(20)30076-X pmid: 32085846 |
| [10] |
Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab[J]. Proc Natl Acad Sci U S A, 2020, 117(20):10970-10975.
doi: 10.1073/pnas.2005615117 URL |
| [11] |
Campins L, Boixeda R, Perez-Cordon L, et al. Early tocilizumab treatment could improve survival among COVID-19 patients[J]. Clin Exp Rheumatol, 2020, 38(3):578.
pmid: 32456769 |
| [12] |
Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study[J]. Lancet Rheumatol, 2020, 2(8):e474-e484.
doi: 10.1016/S2665-9913(20)30173-9 URL |
| [13] |
Jurado A, Martín MC, Abad-Molina C, et al. COVID-19: age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study[J]. Immun Ageing, 2020, 17:22.
doi: 10.1186/s12979-020-00194-w pmid: 32802142 |
| [14] |
Del VD, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival[J]. Nat Med, 2020, 26(10):1636-1643.
doi: 10.1038/s41591-020-1051-9 |
| [15] |
Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19[J]. J Clin Virol, 2020, 127:104370.
doi: 10.1016/j.jcv.2020.104370 URL |
| [16] |
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses[J]. Eur J Epidemiol, 2010, 25(9):603-605.
doi: 10.1007/s10654-010-9491-z pmid: 20652370 |
| [17] |
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample[J]. BMC Med Res Methodol, 2005, 5:13.
pmid: 15840177 |
| [18] |
Liu L, Zheng Y, Cai L, et al. Neutrophil-to-lymphocyte ratio, a critical predictor for assessment of disease severity in patients with COVID-19[J]. Int J Lab Hematol, 2021, 43(2): 329-335.
doi: 10.1111/ijlh.13374 pmid: 33099889 |
| [19] |
Jurado A, Martín MC, Abad-Molina C, et al. COVID-19: Age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study[J]. Immun Ageing, 2020, 17:22.
doi: 10.1186/s12979-020-00194-w pmid: 32802142 |
| [20] |
Ping Y, Xiang Y, Cheng D, et al. Risk factors and clinical features of deterioration in COVID-19 patients in Zhejiang, China: a single-centre, retrospective study[J]. BMC Infect Dis, 2020, 20(1):943.
doi: 10.1186/s12879-020-05682-4 pmid: 33302889 |
| [21] |
Zhang L, Huang B, Xia H, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases[J]. Epidemiol Infect, 2020, 148:e199.
doi: 10.1017/S0950268820002010 URL |
| [22] |
Xiaohong Y, Wanling H, Bing Y, et al. Changes of hematological and immunological parameters in COVID-19 patients[J]. Int J Hematol, 2020, 112 (4):553-559.
doi: 10.1007/s12185-020-02930-w pmid: 32656638 |
| [23] |
Xiuqi W, Wenjuan Z, Jingyu S, et al. Hypolipidemia is associated with the severity of COVID-19[J]. J Clin Lipidol, 2020, 14(3) :297-304.
doi: S1933-2874(20)30078-7 pmid: 32430154 |
| [24] |
Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study[J]. Lancet Haematol, 2020, 7(9):e671-e678.
doi: 10.1016/S2352-3026(20)30217-9 pmid: 32659214 |
| [25] |
Yang G, Changsong W, Kai K, et al. Cytokine storm may not be the chief culprit for the deterioration of COVID-19.[J]. Viral Immunol, 2020, 34(5):336-341.
doi: 10.1089/vim.2020.0243 URL |
| [26] |
Shi P, Ren G, Yang J, et al. Clinical characteristics of imported and second-generation coronavirus disease 2019 (COVID-19) cases in Shaanxi outside Wuhan, China: A multicentre retrospective study[J]. Epidemiol Infect, 2020, 148:e238.
doi: 10.1017/S0950268820002332 URL |
| [27] |
Lv Z, Cheng S, Le J, et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: A retrospective cohort study[J]. Microbes Infect, 2020, 22(4-5):195-199.
doi: S1286-4579(20)30085-X pmid: 32425649 |
| [28] | Weili W, Zhongxiu Z, Xi L, et al. Clinical features and potential risk factors for discerning the critical cases and predicting the outcome of patients with COVID-19[J]. J Clin Lab Anal, 2020, 34(10):e23547. |
| [29] | 孙昀, 孙伟, 叶珺, 等. 168例新型冠状病毒肺炎患者临床特点及重症进展的影响因素分析[J]. 中华急诊医学杂志, 2020, 29(7):901-907. |
| [30] | 杨秀红, 熊蓉, 胡述立, 等. 不同临床分型新型冠状病毒肺炎患者临床特征及抗体、核酸检测结果分析[J]. 实用心脑肺血管病杂志, 2020, 28(9):10-15. |
| [31] | 魏剑浩, 郭倩, 李海聪, 等. 上海地区328例新型冠状病毒肺炎患者实验室数据分析[J]. 检验医学, 2020, 35(8):778-783. |
| [32] | 吕宁, 陆加刚, 罗莎莎, 等. 生化及炎症指标在新型冠状病毒肺炎患者中的变化特征和应用价值[J]. 国际检验医学杂志, 2020, 41(20):2501-2505. |
| [33] | 张永喜, 熊勇, 李新宇, 等. 新型冠状病毒肺炎出院患者203例的临床特点分析[J]. 中华传染病杂志, 2020, 38(8):472-478. |
| [34] | 邹义龙, 余贻汉, 刘尧蓓, 等. 新型冠状病毒肺炎患者病情严重程度与临床特征的关系[J]. 广西医学, 2020, 42(13):1707-1712. |
| [35] | 刘浩, 龙利, 张继波, 等. 新型冠状病毒肺炎患者血清胱抑素C与炎性因子水平变化及其相关性[J]. 天津医药, 2020, 48(8):753-756. |
| [36] |
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China[J]. JAMA, 2020, 323(11):1061-1069.
doi: 10.1001/jama.2020.1585 pmid: 32031570 |
| [37] |
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study[J]. Lancet, 2020, 395(10229):1054-1062.
doi: S0140-6736(20)30566-3 pmid: 32171076 |
| [38] | Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study[J]. BMJ, 2020, 369:m1966. |
| [39] |
Wang Y, Lu X, Li Y, et al. Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19[J]. Am J Respir Crit Care Med, 2020, 201(11):1430-1434.
doi: 10.1164/rccm.202003-0736LE URL |
| [40] |
Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19[J]. Clin Infect Dis, 2021, 73(2):e445-e454.
doi: 10.1093/cid/ciaa954 pmid: 32651997 |
| [41] |
Potere N, Di Nisio M, Cibelli D, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: A case-control study[J]. Ann Rheum Dis, 2021, 80(2):1-2.
doi: 10.1136/annrheumdis-2020-218243 pmid: 32647027 |
| [42] |
Price CC, Altice FL, Shyr Y, et al. Tocilizumab Treatment for Cytokine Release Syndrome in Hospitalized Patients With Coronavirus Disease 2019: Survival and Clinical Outcomes[J]. Chest, 2020, 158(4):1397-1408.
doi: 10.1016/j.chest.2020.06.006 pmid: 32553536 |
| [43] |
Jurado A, Martín MC, Abad-Molina C, et al. COVID-19: age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study[J]. Immun Ageing, 2020, 17:22.
doi: 10.1186/s12979-020-00194-w pmid: 32802142 |
| [44] |
Potere N, Di Nisio M, Cibelli D, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case-control study[J]. Ann Rheum Dis, 2021, 80(2):1-2.
doi: 10.1136/annrheumdis-2020-218243 pmid: 32647027 |
| [1] | 龚财芳, 赵俊宇, 游川. 接纳与承诺疗法对癌症患者心理健康和生活质量影响的meta分析[J]. 临床荟萃, 2024, 39(2): 101-107. |
| [2] | 黄赛虎, 龙中洁, 吴水燕, 柏振江. 新冠疫情前后重症肺炎合并急性呼吸衰竭患儿的临床特点与病原学分析[J]. 临床荟萃, 2024, 39(2): 140-143. |
| [3] | 肖煌怡, 袁建坤, 严梓予, 曾雯姝, 鲁兰莫, 王峻. 认知干预对遗忘型轻度认知障碍老年患者干预效果的meta分析[J]. 临床荟萃, 2024, 39(1): 12-19. |
| [4] | 吕畅, 周利明. TNF-α-308基因多态性与胃癌易感相关性的meta分析[J]. 临床荟萃, 2023, 38(9): 779-787. |
| [5] | 李海, 刘文虎, 彭绍鹏, 王飞. 控制性阶梯式减压术对比快速标准大骨瓣减压术治疗重度颅脑损伤疗效的meta分析[J]. 临床荟萃, 2023, 38(9): 788-795. |
| [6] | 位增, 曹灵, 佘敦敏, 刘彦, 王艳, 张真稳. 54例2型糖尿病患者合并新型冠状病毒感染的死亡原因分析[J]. 临床荟萃, 2023, 38(9): 806-812. |
| [7] | 侯有玲, 李奕, 关红玉, 罗红霞. 目标导向液体治疗在脑肿瘤切除术中应用效果的meta分析[J]. 临床荟萃, 2023, 38(8): 686-693. |
| [8] | 金家辉, 杨阳, 秦铜, 何雨欣, 苏美华. 补充益生菌对2型糖尿病患者糖代谢改善的meta分析[J]. 临床荟萃, 2023, 38(7): 581-587. |
| [9] | 肖王静, 李欣梦, 卢松玲, 孙雪华. 重复经颅磁刺激治疗中枢神经源性吞咽障碍疗效及安全性的meta分析[J]. 临床荟萃, 2023, 38(7): 588-599. |
| [10] | 黄华艳, 林春光, 吴昌儒, 陈永东, 黄焕谋. 新型冠状病毒Omicron变异株与Delta变异株感染患者的临床特征分析[J]. 临床荟萃, 2023, 38(7): 600-605. |
| [11] | 尤奕, 高淑清, 徐浩. 肠内营养对食管癌患者术后临床结局影响的系统综述[J]. 临床荟萃, 2023, 38(6): 485-492. |
| [12] | 沃拉孜汗·玛德尼亚提, 迪力夏提·图尔迪麦麦提, 李梦晨, 拜合提尼沙·吐尔地. 宏基因组二代测序技术在肺结核诊断中应用价值的meta分析[J]. 临床荟萃, 2023, 38(5): 389-398. |
| [13] | 赵哲, 穆培娟, 张冬. 恩度联合顺铂胸腔灌注治疗肺癌合并恶性胸腔积液疗效的meta分析[J]. 临床荟萃, 2023, 38(5): 399-404. |
| [14] | 于雪华, 张宁, 吴婧, 孙惠, 赵云红, 刘改芳. 血清抵抗素、丙二醛、IL-6联合检测对急性胰腺炎严重程度的预测价值[J]. 临床荟萃, 2023, 38(5): 412-416. |
| [15] | 马明福, 魏志国, 何铁英. 急性胰腺炎并发胰腺假性囊肿危险因素的meta分析[J]. 临床荟萃, 2023, 38(4): 293-301. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||