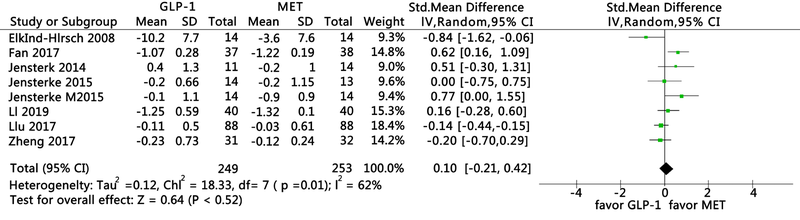
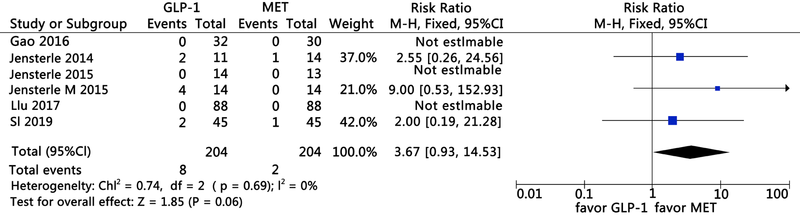
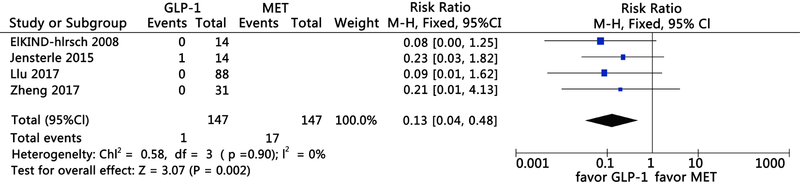
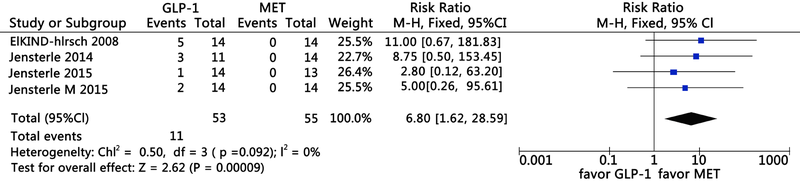
Clinical Focus ›› 2021, Vol. 36 ›› Issue (5): 395-401.doi: 10.3969/j.issn.1004-583X.2021.05.002
Previous Articles Next Articles
Meta-analysis on efficacy and safety of glucagon like peptide-1 receptor agonist on patients with polycystic ovary syndrome
Li Lei, Aaletengqiqige, Zhang Mingchen, Jiang Sheng( )
)
- Department of Endocrinology, First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, China
-
Received:2020-09-30Online:2021-05-20Published:2021-06-09 -
Contact:Jiang Sheng E-mail:xjjsh@126.com
CLC Number:
Cite this article
Li Lei, Aaletengqiqige, Zhang Mingchen, Jiang Sheng. Meta-analysis on efficacy and safety of glucagon like peptide-1 receptor agonist on patients with polycystic ovary syndrome[J]. Clinical Focus, 2021, 36(5): 395-401.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2021.05.002
| 纳入研究 | 纳入人数 (T/C) | 年龄 (岁) | 试验组 | 对照组 | 疗程 (周) | 结局指标 | |||
|---|---|---|---|---|---|---|---|---|---|
| 药物种类 | 剂量/频次 | 药物种类 | 剂量/频次 | ||||||
| Elkind-Hirsch 2008[ | 14/14 | 18~40 | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 1000 mg/bid | 24 | ①②③④⑥⑦⑧ | |
| Jensterle 2014[ | 11/14 | >18 | 利拉鲁肽 | 1.2 mg/qd | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑤⑥⑧ | |
| Jensterle 2015[ | 14/13 | >18 | 利拉鲁肽 | 1.2 mg/qd | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑤⑥⑦⑧ | |
| Jensterle M 2015[ | 14/14 | >18 | 利拉鲁肽 | 1.2 mg/qd | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑤⑧ | |
| Liu 2017[ | 88/88 | 18~40 | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑤⑥⑦ | |
| Zheng 2017[ | 31/32 | 18~40 | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑥⑦ | |
| 范晓霞 2017[ | 37/38 | - | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 500 mg/tid | 12 | ①③④ | |
| 高丽华 2016[ | 32/30 | - | 利拉鲁肽 | 1.2 mg/qd | 二甲双胍 | 500 mg/tid | 12 | ①③⑤ | |
| 李德珍 2019[ | 40/40 | - | 利拉鲁肽 | 1.0 mg/qd | 二甲双胍 | 500 mg/tid | 24 | ③④ | |
| 司晓宁 2019[ | 45/45 | 20~40 | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 500 mg/tid | 24 | ③⑤⑥ | |
| 纳入研究 | 纳入人数 (T/C) | 年龄 (岁) | 试验组 | 对照组 | 疗程 (周) | 结局指标 | |||
|---|---|---|---|---|---|---|---|---|---|
| 药物种类 | 剂量/频次 | 药物种类 | 剂量/频次 | ||||||
| Elkind-Hirsch 2008[ | 14/14 | 18~40 | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 1000 mg/bid | 24 | ①②③④⑥⑦⑧ | |
| Jensterle 2014[ | 11/14 | >18 | 利拉鲁肽 | 1.2 mg/qd | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑤⑥⑧ | |
| Jensterle 2015[ | 14/13 | >18 | 利拉鲁肽 | 1.2 mg/qd | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑤⑥⑦⑧ | |
| Jensterle M 2015[ | 14/14 | >18 | 利拉鲁肽 | 1.2 mg/qd | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑤⑧ | |
| Liu 2017[ | 88/88 | 18~40 | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑤⑥⑦ | |
| Zheng 2017[ | 31/32 | 18~40 | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 1000 mg/bid | 12 | ①②③④⑥⑦ | |
| 范晓霞 2017[ | 37/38 | - | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 500 mg/tid | 12 | ①③④ | |
| 高丽华 2016[ | 32/30 | - | 利拉鲁肽 | 1.2 mg/qd | 二甲双胍 | 500 mg/tid | 12 | ①③⑤ | |
| 李德珍 2019[ | 40/40 | - | 利拉鲁肽 | 1.0 mg/qd | 二甲双胍 | 500 mg/tid | 24 | ③④ | |
| 司晓宁 2019[ | 45/45 | 20~40 | 艾塞那肽 | 10 μg/bid | 二甲双胍 | 500 mg/tid | 24 | ③⑤⑥ | |
| 纳入研究 | 随机方法 | 分配隐藏 | 盲法 | 结果数据的 完整性 | 选择性报告 结果 | 其他偏倚 来源 | |
|---|---|---|---|---|---|---|---|
| 患者与研究者 | 评估者 | ||||||
| Elkind-Hirsch 2008[ | 随机软件 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Jensterle 2014[ | 随机软件 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Jensterle 2015[ | 数字表 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Jensterle M 2015[ | 随机软件 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Liu 2017[ | 不清楚 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Zheng 2017[ | 随机软件 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 范晓霞 2017[ | 不清楚 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 高丽华 2016[ | 数字表 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 李德珍 2019[ | 不清楚 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 司晓宁 2019[ | 不清楚 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 纳入研究 | 随机方法 | 分配隐藏 | 盲法 | 结果数据的 完整性 | 选择性报告 结果 | 其他偏倚 来源 | |
|---|---|---|---|---|---|---|---|
| 患者与研究者 | 评估者 | ||||||
| Elkind-Hirsch 2008[ | 随机软件 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Jensterle 2014[ | 随机软件 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Jensterle 2015[ | 数字表 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Jensterle M 2015[ | 随机软件 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Liu 2017[ | 不清楚 | 不清楚 | 否 | 否 | 完整 | 无 | 不清楚 |
| Zheng 2017[ | 随机软件 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 范晓霞 2017[ | 不清楚 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 高丽华 2016[ | 数字表 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 李德珍 2019[ | 不清楚 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| 司晓宁 2019[ | 不清楚 | 不清楚 | 不清楚 | 不清楚 | 完整 | 无 | 不清楚 |
| [1] |
Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic Ovarian Syndrome (PCOS): Long-term metabolic consequences[J]. Metabolism, 2018, 86:33-43.
doi: 10.1016/j.metabol.2017.09.016 URL |
| [2] |
Escobar-Morreale HF. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment[J]. Nat Rev Endocrinol, 2018, 14(5):270-284.
doi: 10.1038/nrendo.2018.24 pmid: 29569621 |
| [3] |
Macut D, Bjeki'c-Macut J, Raheli'c D, et al. Insulin and the polycystic ovary syndrome[J]. Diabetes Res Clin Pract, 2017, 130:163-170.
doi: 10.1016/j.diabres.2017.06.011 URL |
| [4] |
Practice Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org; Practice Committee of the American Society for Reproductive Medicine. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): a guideline[J]. Fertil Steril, 2017, 108(3):426-441.
doi: 10.1016/j.fertnstert.2017.06.026 URL |
| [5] |
Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: What's new?[J]. Adv Clin Exp Med, 2017, 26(2):359-367.
doi: 10.17219/acem/59380 pmid: 28791858 |
| [6] |
Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1)[J]. Mol Metab, 2019, 30:72-130.
doi: S2212-8778(19)30913-5 pmid: 31767182 |
| [7] |
Nylander M, Frøssing S, Kistorp C, et al. Liraglutide in polycystic ovary syndrome: A randomized trial, investigating effects on thrombogenic potential[J]. Endocr Connect, 2017, 6(2):89-99.
doi: 10.1530/EC-16-0113 pmid: 28119323 |
| [8] |
Frøssing S, Nylander M, Chabanova E, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: A randomized clinical trial[J]. Diabetes Obes Metab, 2018, 20(1):215-218.
doi: 10.1111/dom.13053 pmid: 28681988 |
| [9] | Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. |
| [10] |
Elkind-Hirsch K, Marrioneaux O, Bhushan M, et al. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome[J]. J Clin Endocrinol Metab, 2008, 93(7):2670-2678.
doi: 10.1210/jc.2008-0115 URL |
| [11] |
Jensterle Sever M, Kocjan T, Pfeifer M, et al. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin[J]. Eur J Endocrinol, 2014, 170(3):451-459.
doi: 10.1530/EJE-13-0797 pmid: 24362411 |
| [12] |
Jensterle M, Salamun V, Kocjan T, et al. Short term monotherapy with GLP-1 receptor agonist liraglutide or PDE 4 inhibitor roflumilast is superior to metformin in weight loss in obese PCOS women: A pilot randomized study[J]. J Ovarian Res, 2015, 8:32.
doi: 10.1186/s13048-015-0161-3 pmid: 26032655 |
| [13] |
Jensterle M, Kravos NA, Pfeifer M, et al. A 12-week treatment with the long-acting glucagon-like peptide 1 receptor agonist liraglutide leads to significant weight loss in a subset of obese women with newly diagnosed polycystic ovary syndrome[J]. Hormones (Athens), 2015, 14(1):81-90.
pmid: 25885106 |
| [14] |
Liu X, Zhang Y, Zheng SY, et al. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome[J]. Clin Endocrinol (Oxf), 2017, 87(6):767-774.
doi: 10.1111/cen.2017.87.issue-6 URL |
| [15] |
Zheng S, Zhang Y, Long T, et al. Short term monotherapy with exenatide is superior to metformin in weight loss, improving insulin resistance and inflammation in Chinese overweight/obese PCOS women[J]. Obesity Medicine, 2017, 7:15-20.
doi: 10.1016/j.obmed.2017.06.003 URL |
| [16] | 范晓霞, 姚勇利, 胡耀嘉, 等. 艾塞那肽对高海拔地区2型糖尿病伴多囊卵巢综合征患者治疗作用的观察[J]. 中国糖尿病杂志, 2017, 25(3):241-244. |
| [17] | 高丽华, 张彩兰, 刘旭阳, 等. 利拉鲁肽治疗多囊卵巢综合征合并2型糖尿病患者的初步临床研究[J]. 临床荟萃, 2016, 31(5):539-542. |
| [18] | 李德珍. 利拉鲁肽对糖尿病合并多囊卵巢综合征患者内分泌代谢的影响[J]. 实用糖尿病杂志, 2019, 15(6):13. |
| [19] | 司晓宁, 马晶. 艾塞那肽对多囊卵巢综合征患者子宫内膜胰岛素抵抗及内分泌代谢的影响[J]. 中国妇幼保健, 2019, 34(3):547-549. |
| [20] |
Jin P, Xie Y. Treatment strategies for women with polycystic ovary syndrome[J]. Gynecol Endocrinol, 2018, 34(4):272-277.
doi: 10.1080/09513590.2017.1395841 URL |
| [21] |
Morgante G, Massaro MG, Di Sabatino A, et al. Therapeutic approach for metabolic disorders and infertility in women with PCOS[J]. Gynecol Endocrinol, 2018, 34(1):4-9.
doi: 10.1080/09513590.2017.1370644 pmid: 28850273 |
| [22] |
Fruzzetti F, Perini D, Russo M, et al. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS)[J]. Gynecol Endocrinol, 2017, 33(1):39-42.
doi: 10.1080/09513590.2016.1236078 pmid: 27808588 |
| [23] |
Lamos EM, Malek R, Davis SN. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome[J]. Expert Rev Clin Pharmacol, 2017, 10(4):401-408.
doi: 10.1080/17512433.2017.1292125 URL |
| [24] |
Zhou J, Massey S, Story D, et al. Metformin: An old drug with new applications[J]. Int J Mol Sci, 2018, 19(10):2863.
doi: 10.3390/ijms19102863 URL |
| [25] |
Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies[J]. Mol Metab, 2020, 35:100937.
doi: S2212-8778(20)30003-X pmid: 32244180 |
| [26] |
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man[J]. Diabetologia, 1985, 28(7):412-419.
pmid: 3899825 |
| [27] |
Battaglia C, Battaglia B, Casadio P, et al. Metformin metabolic and vascular effects in normal weight hyperinsulinemic polycystic ovary syndrome patients treated with contraceptive vaginal ring. A pilot study[J]. Gynecol Endocrinol, 2020, 36(12):1062-1069.
doi: 10.1080/09513590.2020.1770213 URL |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||