Clinical Focus ›› 2022, Vol. 37 ›› Issue (12): 1074-1080.doi: 10.3969/j.issn.1004-583X.2022.12.002
Previous Articles Next Articles
Efficacy and safety of PCSK9 inhibitors on atherosclerotic cardiovascular disease: A meta-analysis
Zhang Lei, Lou Haidong, Zhi Yu, Qi Shuying( )
)
- Department of Cardiology,Boao Super Hospital,Qionghai 571437,China
-
Received:2022-08-09Online:2022-12-20Published:2023-01-18 -
Contact:Qi Shuying E-mail:qishy@sina.com
CLC Number:
Cite this article
Zhang Lei, Lou Haidong, Zhi Yu, Qi Shuying. Efficacy and safety of PCSK9 inhibitors on atherosclerotic cardiovascular disease: A meta-analysis[J]. Clinical Focus, 2022, 37(12): 1074-1080.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2022.12.002
| 纳入研究 | 研究分组 | 纳入标准 | 样本量 (例) | 给药方案 | 高强度他汀(%) | 年龄(岁) | 男性(%) | 基线LDL-C 水平(mg/dl) | 随访 时间 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | |||||||||
| ODYSSEY COMBO I 2015[ | Ali vs安慰剂 | ASCVD或ASCVD等危 | 316 | 75 mg Q2W(若8周时LDL-C≥70 mg/dl,12周时给予150 mg q2w) | 61.7 | 64.5 | 63 | 63 | 62.7 | 72 | 94.8 | 100.2 | 52周 | |||
| ODYSSEY COMBO II 2015[ | Ali vs依折麦布 | CHD或CHD等危 | 720 | 75 mg Q2W(若8周时LDL-C≥70 mg/dl,12周时给予150 mg q2w) | 66.8 | 66.4 | 61.7 | 61.3 | 75.2 | 70.5 | 108.3 | 104 | 52周 | |||
| ODYSSEY LONG TERM 2015[ | Ali vs安慰剂 | 杂合子FH或CHD或CHD等危 | 2341 | 150 mg q2w | 46.8 | 46.7 | 60.4 | 60.6 | 63.3 | 60.2 | 122.7 | 121.9 | 78周 | |||
| ODYSSEY OUTCOMES 2018[ | Ali vs安慰剂 | ACS | 18, 924 | 75 mg q2w | 88.6 | 89.1 | 58.5 | 58.6 | 74.7 | 74.9 | 92 | 92 | 2.8年 | |||
| PACMAN-AMI 2022[ | Ali vs安慰剂 | AMI,适合冠状动脉腔内成像 | 300 | 150 mg q2w 瑞舒伐他汀20 mg qd | 7.4 | 5.9 | 58.4 | 58.6 | 83.8 | 78.3 | 154.8 | 150.9 | 52周 | |||
| GLAGOV 2016[ | Evo vs安慰剂 | 冠状动脉造影证实冠状动脉狭窄 | 968 | 420 mg q4w | 57.9 | 59.9 | 59.8 | 59.8 | 72.1 | 72.3 | 92.6 | 92.4 | 78周 | |||
| FOURIER 2017[ | Evo vs安慰剂 | ASCVD | 27, 564 | 140 mg q2w或420 mg q4w | 69.5 | 69.1 | 62.5 | 62.5 | 75.4 | 75.5 | 92 | 92 | 2.2年 | |||
| HUYGENS 2022[ | Evo vs安慰剂 | NSTEMI,适合冠状动脉腔内成像 | 161 | 420 mg q4w | 82.7 | 78.8 | 60.9 | 60.2 | 75 | 67.9 | 140.4 | 142.1 | 52周 | |||
| ORION-10 2020[ | Inc vs安慰剂 | ASCVD | 1561 | 284 mg,第1天、第90天,然后每6个月 | 67.2 | 68.8 | 66.4 | 65.7 | 68.5 | 70.3 | 104.5 | 104.8 | 540天 | |||
| ORION-11 2020[ | Inc vs安慰剂 | ASCVD或ASCVD等危 | 1617 | 284 mg,第1天、第90天,然后每6个月 | 79.0 | 78.2 | 64.8 | 64.8 | 71.5 | 72 | 107.2 | 103.7 | 540天 | |||
| 纳入研究 | 研究分组 | 纳入标准 | 样本量 (例) | 给药方案 | 高强度他汀(%) | 年龄(岁) | 男性(%) | 基线LDL-C 水平(mg/dl) | 随访 时间 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | 干预组 | 对照组 | |||||||||
| ODYSSEY COMBO I 2015[ | Ali vs安慰剂 | ASCVD或ASCVD等危 | 316 | 75 mg Q2W(若8周时LDL-C≥70 mg/dl,12周时给予150 mg q2w) | 61.7 | 64.5 | 63 | 63 | 62.7 | 72 | 94.8 | 100.2 | 52周 | |||
| ODYSSEY COMBO II 2015[ | Ali vs依折麦布 | CHD或CHD等危 | 720 | 75 mg Q2W(若8周时LDL-C≥70 mg/dl,12周时给予150 mg q2w) | 66.8 | 66.4 | 61.7 | 61.3 | 75.2 | 70.5 | 108.3 | 104 | 52周 | |||
| ODYSSEY LONG TERM 2015[ | Ali vs安慰剂 | 杂合子FH或CHD或CHD等危 | 2341 | 150 mg q2w | 46.8 | 46.7 | 60.4 | 60.6 | 63.3 | 60.2 | 122.7 | 121.9 | 78周 | |||
| ODYSSEY OUTCOMES 2018[ | Ali vs安慰剂 | ACS | 18, 924 | 75 mg q2w | 88.6 | 89.1 | 58.5 | 58.6 | 74.7 | 74.9 | 92 | 92 | 2.8年 | |||
| PACMAN-AMI 2022[ | Ali vs安慰剂 | AMI,适合冠状动脉腔内成像 | 300 | 150 mg q2w 瑞舒伐他汀20 mg qd | 7.4 | 5.9 | 58.4 | 58.6 | 83.8 | 78.3 | 154.8 | 150.9 | 52周 | |||
| GLAGOV 2016[ | Evo vs安慰剂 | 冠状动脉造影证实冠状动脉狭窄 | 968 | 420 mg q4w | 57.9 | 59.9 | 59.8 | 59.8 | 72.1 | 72.3 | 92.6 | 92.4 | 78周 | |||
| FOURIER 2017[ | Evo vs安慰剂 | ASCVD | 27, 564 | 140 mg q2w或420 mg q4w | 69.5 | 69.1 | 62.5 | 62.5 | 75.4 | 75.5 | 92 | 92 | 2.2年 | |||
| HUYGENS 2022[ | Evo vs安慰剂 | NSTEMI,适合冠状动脉腔内成像 | 161 | 420 mg q4w | 82.7 | 78.8 | 60.9 | 60.2 | 75 | 67.9 | 140.4 | 142.1 | 52周 | |||
| ORION-10 2020[ | Inc vs安慰剂 | ASCVD | 1561 | 284 mg,第1天、第90天,然后每6个月 | 67.2 | 68.8 | 66.4 | 65.7 | 68.5 | 70.3 | 104.5 | 104.8 | 540天 | |||
| ORION-11 2020[ | Inc vs安慰剂 | ASCVD或ASCVD等危 | 1617 | 284 mg,第1天、第90天,然后每6个月 | 79.0 | 78.2 | 64.8 | 64.8 | 71.5 | 72 | 107.2 | 103.7 | 540天 | |||
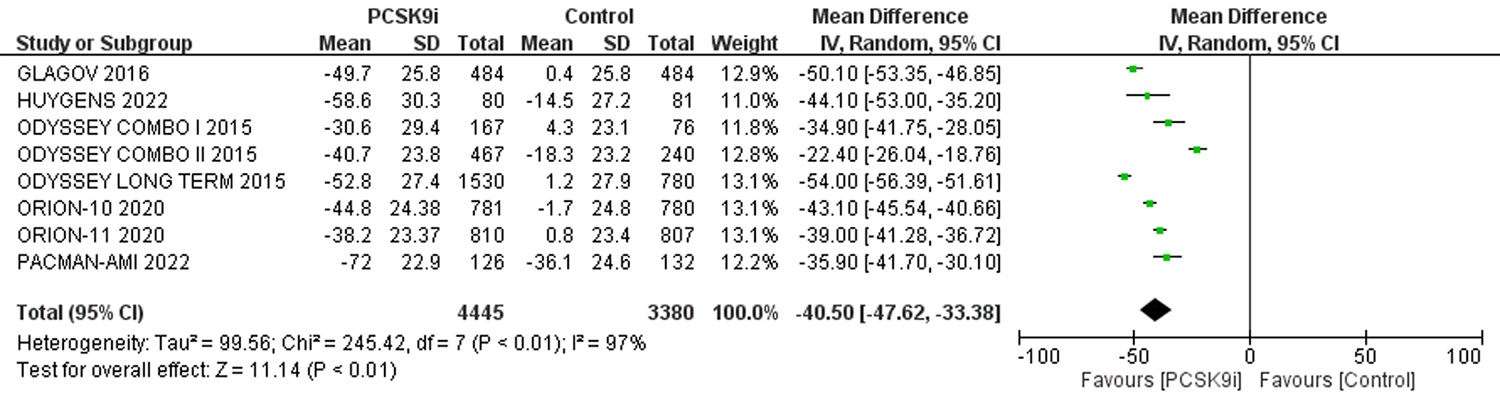
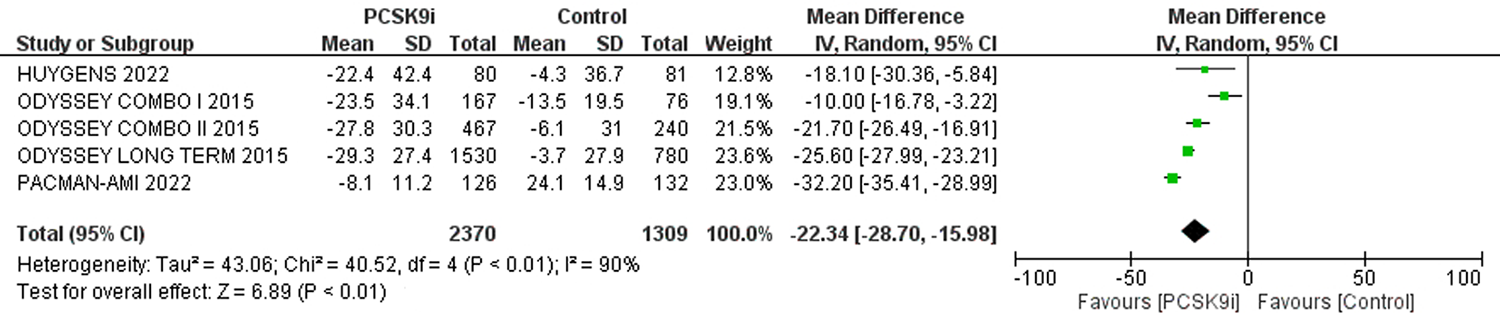
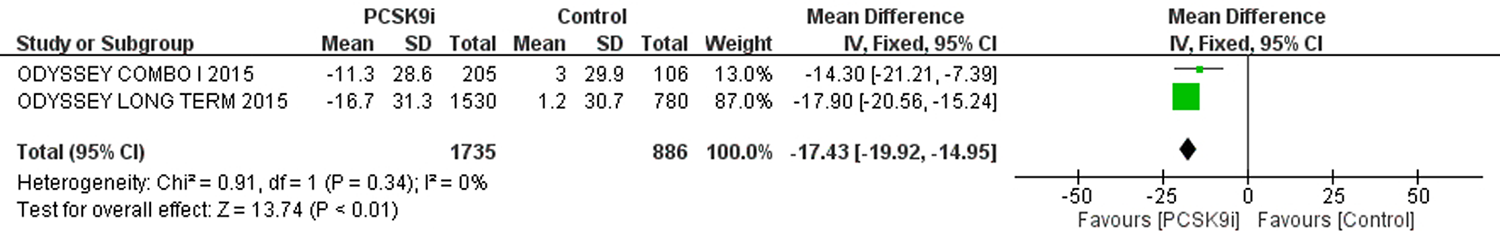
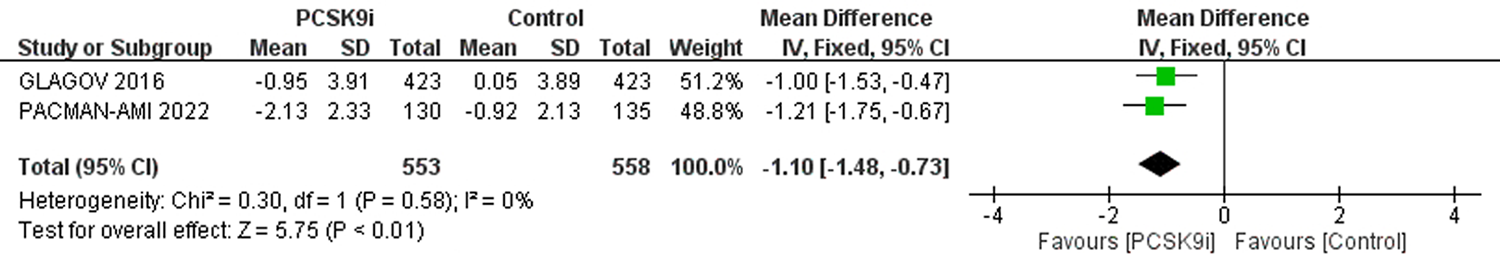
| 评价指标 | 纳入文献 | 样本量 | 异质性检验 | 效应模型 | 合并效应值 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCSK9i | 对照组 | P值 | 95%CI | P值 | ||||||
| 严重不良反应 | [3-6, 8, 9, 11] | 26751 | 25623 | 0 | 0.63 | 固定 | 0.97 | 0.95,1.00 | 0.10 | |
| 注射部位反应 | [3-11] | 26948 | 25829 | 15% | 0.31 | 固定 | 1.57 | 1.40,1.76 | <0.01 | |
| 肌痛 | [3-5, 8-11] | 17380 | 16261 | 18% | 0.29 | 固定 | 1.07 | 0.97,1.18 | 0.17 | |
| 神经认知反应 | [3-9] | 26087 | 24970 | 24% | 0.25 | 固定 | 1.00 | 0.87,1.15 | 0.96 | |
| 新发糖尿病 | [5, 6, 8, 9, 11] | 17398 | 16914 | 0 | 0.44 | 固定 | 1.01 | 0.95,1.08 | 0.75 | |
| ALT>3ULN | [4-6, 11] | 12964 | 11942 | 0 | 0.74 | 固定 | 0.94 | 0.79,1.12 | 0.50 | |
| AST>3ULN | [5, 6, 11] | 12492 | 11699 | 0 | 0.52 | 固定 | 0.91 | 0.74,1.11 | 0.33 | |
| CK>3ULN或 5UL或10ULN | [4-6, 8, 9, 11] | 26962 | 25934 | 0 | 0.94 | 固定 | 0.93 | 0.78,1.12 | 0.47 | |
| 评价指标 | 纳入文献 | 样本量 | 异质性检验 | 效应模型 | 合并效应值 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCSK9i | 对照组 | P值 | 95%CI | P值 | ||||||
| 严重不良反应 | [3-6, 8, 9, 11] | 26751 | 25623 | 0 | 0.63 | 固定 | 0.97 | 0.95,1.00 | 0.10 | |
| 注射部位反应 | [3-11] | 26948 | 25829 | 15% | 0.31 | 固定 | 1.57 | 1.40,1.76 | <0.01 | |
| 肌痛 | [3-5, 8-11] | 17380 | 16261 | 18% | 0.29 | 固定 | 1.07 | 0.97,1.18 | 0.17 | |
| 神经认知反应 | [3-9] | 26087 | 24970 | 24% | 0.25 | 固定 | 1.00 | 0.87,1.15 | 0.96 | |
| 新发糖尿病 | [5, 6, 8, 9, 11] | 17398 | 16914 | 0 | 0.44 | 固定 | 1.01 | 0.95,1.08 | 0.75 | |
| ALT>3ULN | [4-6, 11] | 12964 | 11942 | 0 | 0.74 | 固定 | 0.94 | 0.79,1.12 | 0.50 | |
| AST>3ULN | [5, 6, 11] | 12492 | 11699 | 0 | 0.52 | 固定 | 0.91 | 0.74,1.11 | 0.33 | |
| CK>3ULN或 5UL或10ULN | [4-6, 8, 9, 11] | 26962 | 25934 | 0 | 0.94 | 固定 | 0.93 | 0.78,1.12 | 0.47 | |
| [1] | 《中国心血管健康与疾病报告》编写组. 《中国心血管健康与疾病报告2020》概述[J]. 中国心血管病研究, 2021, 19(7):582-590. |
| [2] | 中国心血管健康与疾病报告编写组. 中国心血管健康与疾病报告2021概要[J]. 中国循环杂志, 2022, 37(6):553-578. |
| [3] |
Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study[J]. Am Heart J, 2015, 169(6):906-915.e13.
doi: 10.1016/j.ahj.2015.03.004 pmid: 26027630 |
| [4] |
Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: The ODYSSEY COMBO II randomized controlled trial[J]. Eur Heart J, 2015, 36(19):1186-94.
doi: 10.1093/eurheartj/ehv028 pmid: 25687353 |
| [5] |
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events[J]. N Engl J Med, 2015, 372(16):1489-1499.
doi: 10.1056/NEJMoa1501031 URL |
| [6] |
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome[J]. N Engl J Med, 2018, 379(22):2097-2107.
doi: 10.1056/NEJMoa1801174 URL |
| [7] |
Räber L, Ueki Y, Otsuka T, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: The pacman-ami randomized clinical trial[J]. JAMA, 2022, 327(18):1771-1781.
doi: 10.1001/jama.2022.5218 pmid: 35368058 |
| [8] |
Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial[J]. JAMA, 2016, 316(22):2373-2384.
doi: 10.1001/jama.2016.16951 pmid: 27846344 |
| [9] |
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease[J]. N Engl J Med, 2017, 376(18):1713-1722.
doi: 10.1056/NEJMoa1615664 URL |
| [10] |
Nicholls SJ, Kataoka Y, Nissen SE, et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction[J]. JACC Cardiovasc Imaging, 2022, 15(7):1308-1321.
doi: 10.1016/j.jcmg.2022.03.002 URL |
| [11] |
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol[J]. N Engl J Med, 2020, 382(16):1507-1519.
doi: 10.1056/NEJMoa1912387 URL |
| [12] |
Geng Q, Li X, Sun Q, et al. Efficacy and safety of PCSK9 inhibition in cardiovascular disease: A meta-analysis of 45 randomized controlled trials[J]. Cardiol J, 2022, 29(4):574-581.
doi: 10.5603/CJ.a2021.0110 URL |
| [13] |
Vicente-Valor J, García-González X, Ibáñez-García S, et al. PCSK9 inhibitors revisited: Effectiveness and safety of PCSK9 inhibitors in a real-life Spanish cohort[J]. Biomed Pharmacother, 2022, 146:112519.
doi: 10.1016/j.biopha.2021.112519 URL |
| [14] |
Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease[J]. Am Heart J, 2008, 155(4):772-779.
doi: 10.1016/j.ahj.2007.12.011 pmid: 18371492 |
| [15] | Guedeney P, Giustino G, Sorrentino S, et al. Efficacy and safety of alirocumab and evolocumab: A systematic review and meta-analysis of randomized controlled trials[J]. Eur Heart J, 2019,ehz430. |
| [16] |
Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque[J]. J Am Coll Cardiol, 2006, 47(8 Suppl):C13-18.
doi: 10.1016/j.jacc.2005.10.065 pmid: 16631505 |
| [17] |
Gencer B, Mach F, Guo J, et al. Cognition after lowering LDL-cholesterol with evolocumab[J]. J Am Coll Cardiol, 2020, 75(18):2283-2293.
doi: S0735-1097(20)34654-4 pmid: 32381158 |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 106
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 353
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||