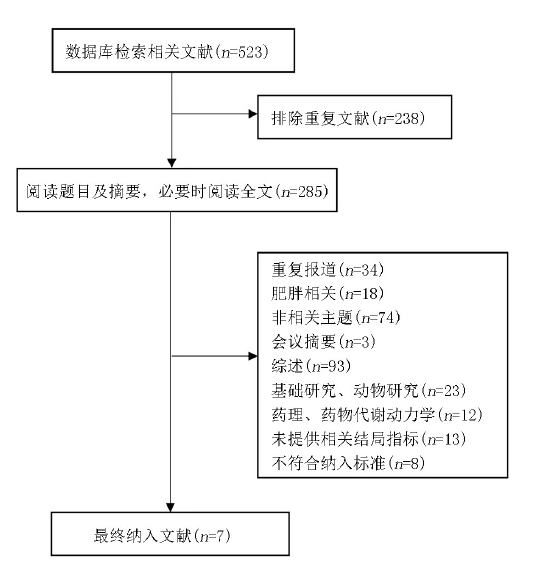
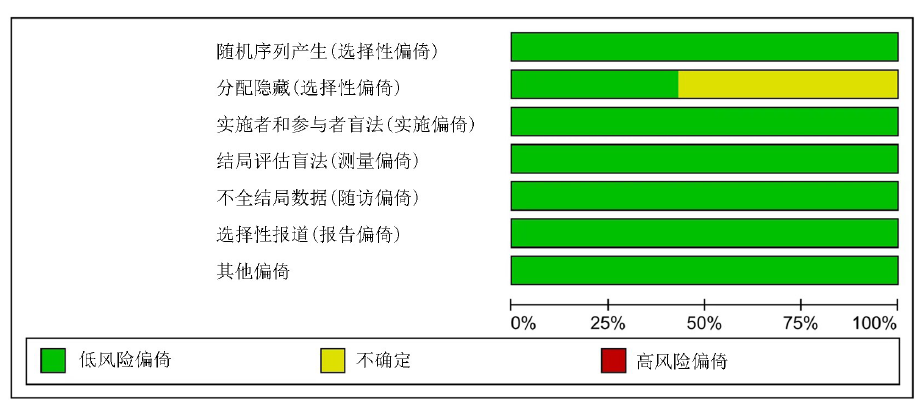
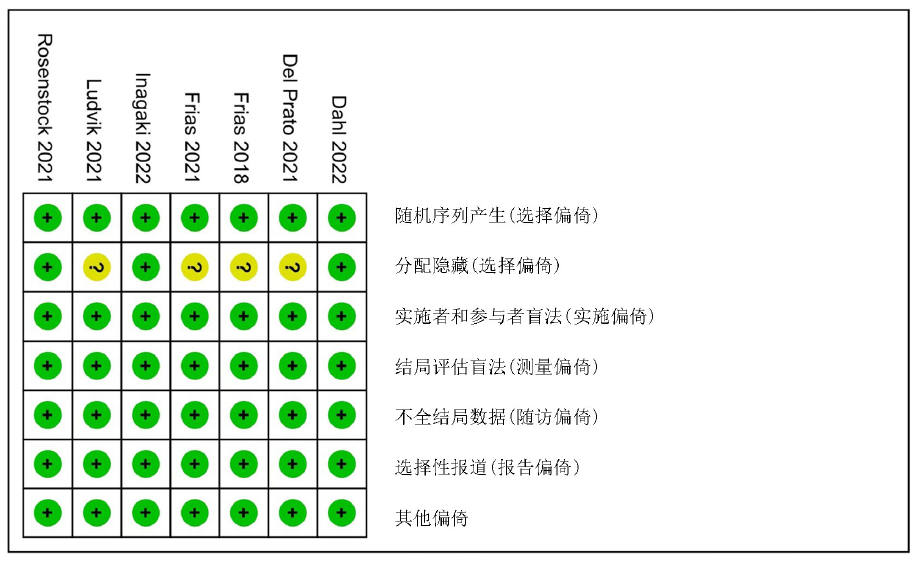
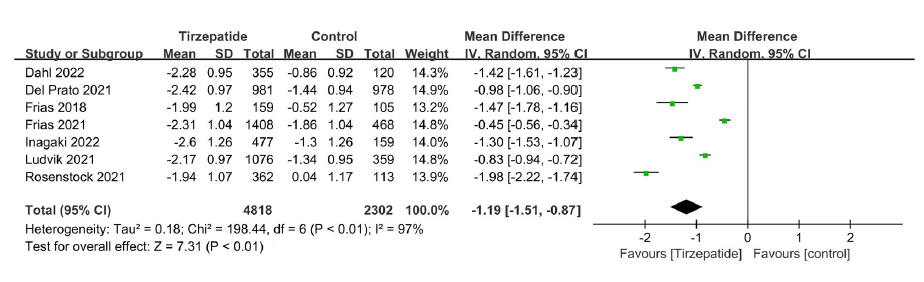
Clinical Focus ›› 2023, Vol. 38 ›› Issue (1): 20-36.doi: 10.3969/j.issn.1004-583X.2023.01.002
Previous Articles Next Articles
Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: A meta-analysis
- 1. Department of Clinical Nutrition,Liuzhou Worker's Hospital,Liuzhou 545000,China
2. First College of Clinical Medicine,Guangxi Medical University,Nanning 530000,China
-
Received:2022-08-02Online:2023-01-20Published:2023-03-03 -
Contact:Zhang Weijian E-mail:cheungweijian@163.com
CLC Number:
Cite this article
Xie Feifei, Zhang Weijian. Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: A meta-analysis[J]. Clinical Focus, 2023, 38(1): 20-36.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2023.01.002
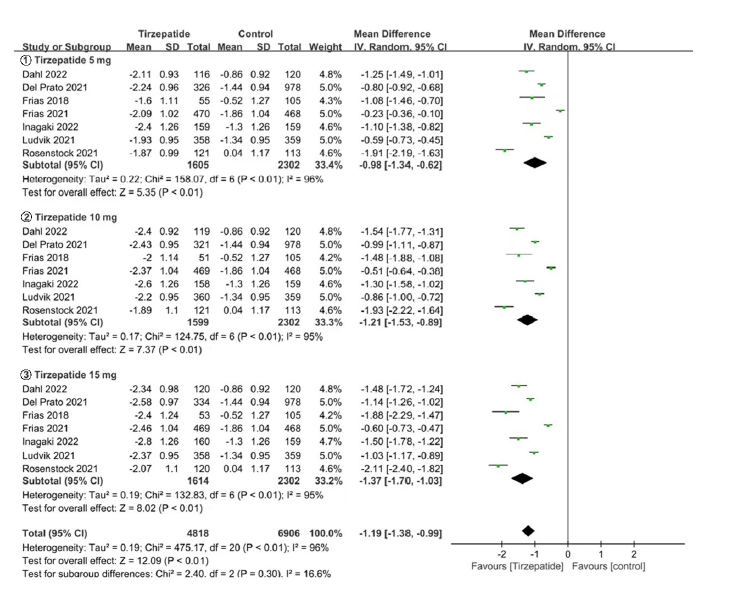
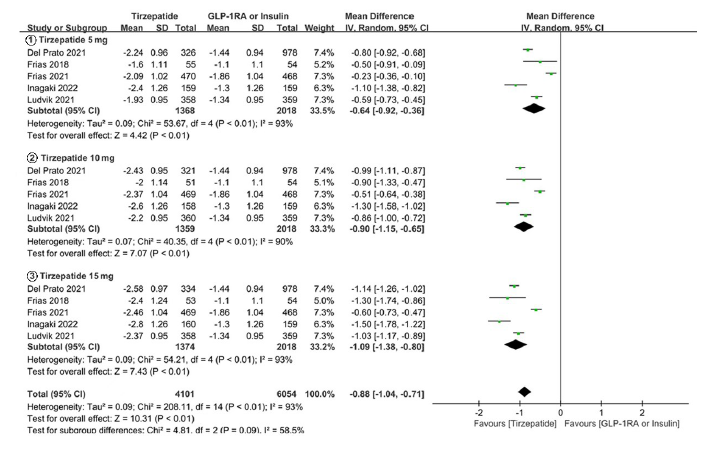
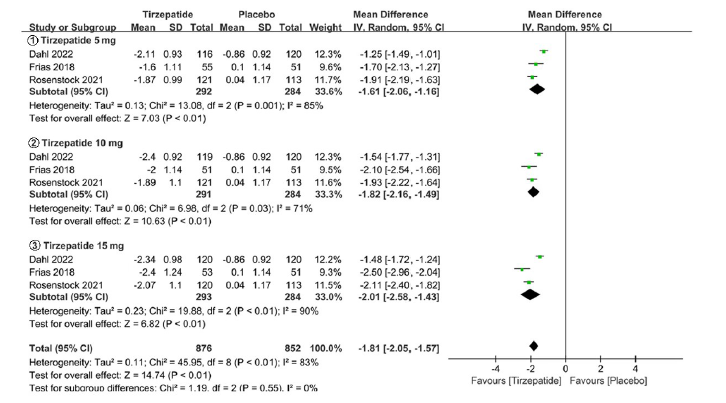
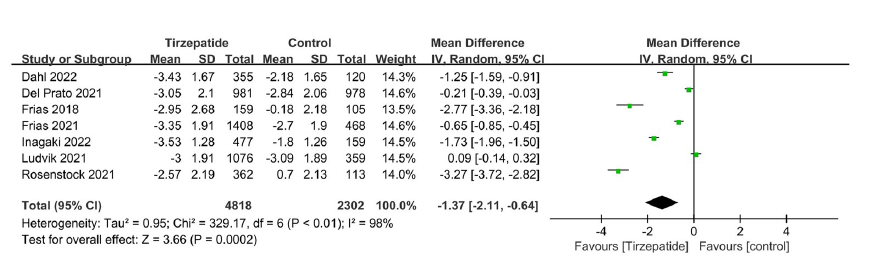
Tab. 1 Basic information of included studies
| 纳入研究 | 干预措施 | 样本量(例) | 年龄(岁) | HbA1c(%) | 疗程(周) | 质量等级 |
|---|---|---|---|---|---|---|
| Frias 2018[ | Tirzepatide 5 mg | 55 | 57.9±8.2 | 8.2±1.0 | ||
| Tirzepatide 10 mg | 51 | 56.5±9.9 | 8.2±1.1 | |||
| Tirzepatide 15 mg | 53 | 56.0±7.6 | 8.1±1.1 | 26 | B | |
| Dulaglutide 1.5 mg | 54 | 58.7±7.8 | 8.1±1.0 | |||
| Placebo | 51 | 56.6±8.9 | 8.0±0.9 | |||
| Rosenstock 2021[ | Tirzepatide 5 mg | 121 | 54.1±11.9 | 8.0±0.8 | ||
| Tirzepatide 10 mg | 121 | 55.8±10.4 | 7.9±0.8 | 40 | A | |
| Tirzepatide 15 mg | 121 | 52.9±12.3 | 7.9±1.0 | |||
| Placebo | 115 | 53.6±12.8 | 8.1±0.8 | |||
| Frías 2021[ | Tirzepatide 5 mg | 470 | 56.3±10.0 | 8.3±1.1 | ||
| Tirzepatide 10 mg | 469 | 57.2±10.5 | 8.3±1.0 | 40 | B | |
| Tirzepatide 15 mg | 470 | 55.9±10.4 | 8.3±1.0 | |||
| Semaglutide 1 mg | 469 | 56.9±10.8 | 8.3±1.0 | |||
| Ludvik 2021[ | Tirzepatide 5 mg | 358 | 57.2±10.1 | 8.2±0.9 | ||
| Tirzepatide 10 mg | 360 | 57.4±9.7 | 8.2±0.9 | 52 | B | |
| Tirzepatide 15 mg | 359 | 57.5±10.2 | 8.2±0.9 | |||
| Insulin degludec | 360 | 57.5±10.1 | 8.1±0.9 | |||
| Del Prato 2021[ | Tirzepatide 5 mg | 329 | 62.9±8.6 | 8.5±0.8 | ||
| Tirzepatide 10 mg | 328 | 63.7±8.7 | 8.6±0.9 | 52 | B | |
| Tirzepatide 15 mg | 338 | 63.7±8.6 | 8.5±1.0 | |||
| Insulin glargine | 1 000 | 63.8±8.5 | 8.5±0.9 | |||
| Dahl 2022[ | Tirzepatide 5 mg | 116 | 62±10 | 8.3±0.9 | ||
| Tirzepatide 10 mg | 119 | 60±10 | 8.4±0.8 | 40 | A | |
| Tirzepatide 15 mg | 120 | 61±10 | 8.2±0.9 | |||
| Placebo | 120 | 60±10 | 8.4±0.8 | |||
| Inagaki 2022[ | Tirzepatide 5 mg | 159 | 56.8±10.1 | 8.2±0.9 | ||
| Tirzepatide 10 mg | 158 | 56.2±10.3 | 8.2±0.9 | 52 | A | |
| Tirzepatide 15 mg | 160 | 56.0±10.7 | 8.2±0.9 | |||
| Dulaglutide 0.75 mg | 159 | 57.5±10.2 | 8.2±0.9 |
Tab. 1 Basic information of included studies
| 纳入研究 | 干预措施 | 样本量(例) | 年龄(岁) | HbA1c(%) | 疗程(周) | 质量等级 |
|---|---|---|---|---|---|---|
| Frias 2018[ | Tirzepatide 5 mg | 55 | 57.9±8.2 | 8.2±1.0 | ||
| Tirzepatide 10 mg | 51 | 56.5±9.9 | 8.2±1.1 | |||
| Tirzepatide 15 mg | 53 | 56.0±7.6 | 8.1±1.1 | 26 | B | |
| Dulaglutide 1.5 mg | 54 | 58.7±7.8 | 8.1±1.0 | |||
| Placebo | 51 | 56.6±8.9 | 8.0±0.9 | |||
| Rosenstock 2021[ | Tirzepatide 5 mg | 121 | 54.1±11.9 | 8.0±0.8 | ||
| Tirzepatide 10 mg | 121 | 55.8±10.4 | 7.9±0.8 | 40 | A | |
| Tirzepatide 15 mg | 121 | 52.9±12.3 | 7.9±1.0 | |||
| Placebo | 115 | 53.6±12.8 | 8.1±0.8 | |||
| Frías 2021[ | Tirzepatide 5 mg | 470 | 56.3±10.0 | 8.3±1.1 | ||
| Tirzepatide 10 mg | 469 | 57.2±10.5 | 8.3±1.0 | 40 | B | |
| Tirzepatide 15 mg | 470 | 55.9±10.4 | 8.3±1.0 | |||
| Semaglutide 1 mg | 469 | 56.9±10.8 | 8.3±1.0 | |||
| Ludvik 2021[ | Tirzepatide 5 mg | 358 | 57.2±10.1 | 8.2±0.9 | ||
| Tirzepatide 10 mg | 360 | 57.4±9.7 | 8.2±0.9 | 52 | B | |
| Tirzepatide 15 mg | 359 | 57.5±10.2 | 8.2±0.9 | |||
| Insulin degludec | 360 | 57.5±10.1 | 8.1±0.9 | |||
| Del Prato 2021[ | Tirzepatide 5 mg | 329 | 62.9±8.6 | 8.5±0.8 | ||
| Tirzepatide 10 mg | 328 | 63.7±8.7 | 8.6±0.9 | 52 | B | |
| Tirzepatide 15 mg | 338 | 63.7±8.6 | 8.5±1.0 | |||
| Insulin glargine | 1 000 | 63.8±8.5 | 8.5±0.9 | |||
| Dahl 2022[ | Tirzepatide 5 mg | 116 | 62±10 | 8.3±0.9 | ||
| Tirzepatide 10 mg | 119 | 60±10 | 8.4±0.8 | 40 | A | |
| Tirzepatide 15 mg | 120 | 61±10 | 8.2±0.9 | |||
| Placebo | 120 | 60±10 | 8.4±0.8 | |||
| Inagaki 2022[ | Tirzepatide 5 mg | 159 | 56.8±10.1 | 8.2±0.9 | ||
| Tirzepatide 10 mg | 158 | 56.2±10.3 | 8.2±0.9 | 52 | A | |
| Tirzepatide 15 mg | 160 | 56.0±10.7 | 8.2±0.9 | |||
| Dulaglutide 0.75 mg | 159 | 57.5±10.2 | 8.2±0.9 |
| [1] |
Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): A randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial[J]. Lancet Diabetes Endocrinol, 2017, 5(5):355-366.
doi: 10.1016/S2213-8587(17)30085-2 URL |
| [2] | Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial[J]. Lancet, 2018, 392(10160):2180-2193. |
| [3] | Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions[M]. John Wiley & Sons, Ltd, 2019. |
| [4] | Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial[J]. Lancet, 2021, 398(10295):143-155. |
| [5] |
Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes[J]. N Engl J Med, 2021, 385(6):503-515.
doi: 10.1056/NEJMoa2107519 URL |
| [6] |
Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial [J]. Lancet, 2021, 398(10300):583-598.
doi: 10.1016/S0140-6736(21)01443-4 pmid: 34370970 |
| [7] |
Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial[J]. Lancet, 2021, 398(10313):1811-1824.
doi: 10.1016/S0140-6736(21)02188-7 pmid: 34672967 |
| [8] |
Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: The SURPASS-5 randomized clinical trial[J]. JAMA, 2022, 327(6):534-545.
doi: 10.1001/jama.2022.0078 pmid: 35133415 |
| [9] |
Inagaki N, Takeuchi M, Oura T, et al. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): A double-blind, multicentre, randomised, phase 3 trial[J]. Lancet Diabetes Endocrinol, 2022, 10(9):623-633.
doi: 10.1016/S2213-8587(22)00188-7 URL |
| [10] |
Zhao FH, Zhou QT, Cong ZT, et al. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors[J]. Nat Commun, 2022, 13(1): 1057.
doi: 10.1038/s41467-022-28683-0 pmid: 35217653 |
| [11] |
Gribble FM, Reimann F. metabolic Messengers: Glucagon-like peptide 1[J]. Nat metab, 2021, 3(2): 142-148.
doi: 10.1038/s42255-020-00327-x pmid: 33432200 |
| [12] |
Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept[J]. Mol metab, 2018, 18:3-14.
doi: S2212-8778(18)30900-1 pmid: 30473097 |
| [13] |
Samms RJ, Coghlan MP, Sloop KW. How may GIP enhance the therapeutic efficacy of GLP-1?[J]. Trends Endocrinol metab, 2020, 31(6): 410-421.
doi: 10.1016/j.tem.2020.02.006 URL |
| [14] |
Samms RJ, Christe ME, Collins KA, et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice[J]. J Clin Invest, 2021, 131(12):e146353.
doi: 10.1172/JCI146353 URL |
| [15] |
Thomas MK, Nikooienejad A, Bray R, et al. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes[J]. J Clin Endocrinol Metab, 2021, 106(2):388-396.
doi: 10.1210/clinem/dgaa863 pmid: 33236115 |
| [16] |
Heise T, Mari A, DeVries JH, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: A multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial[J]. Lancet Diabetes Endocrinol, 2022, 10(6):418-429.
doi: 10.1016/S2213-8587(22)00085-7 URL |
| [17] | Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans[J]. Sci Transl Med, 2013, 5(209):209ra151. |
| [18] |
Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity[J]. N Engl J Med, 2022, 387(3):205-216.
doi: 10.1056/NEJMoa2206038 URL |
| [19] |
Tanday N, Flatt PR, Irwin N. metabolic responses and benefits of glucagon-like peptide-1(GLP-1) receptor ligands[J]. Br J Pharmacol, 2022, 179(4):526-541.
doi: 10.1111/bph.15485 URL |
| [20] |
Tan TM, Khoo B. Tirzepatide and the new era of twincretins for diabetes[J]. Lancet, 2021, 398(10295): 95-97.
doi: 10.1016/S0140-6736(21)01390-8 URL |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||