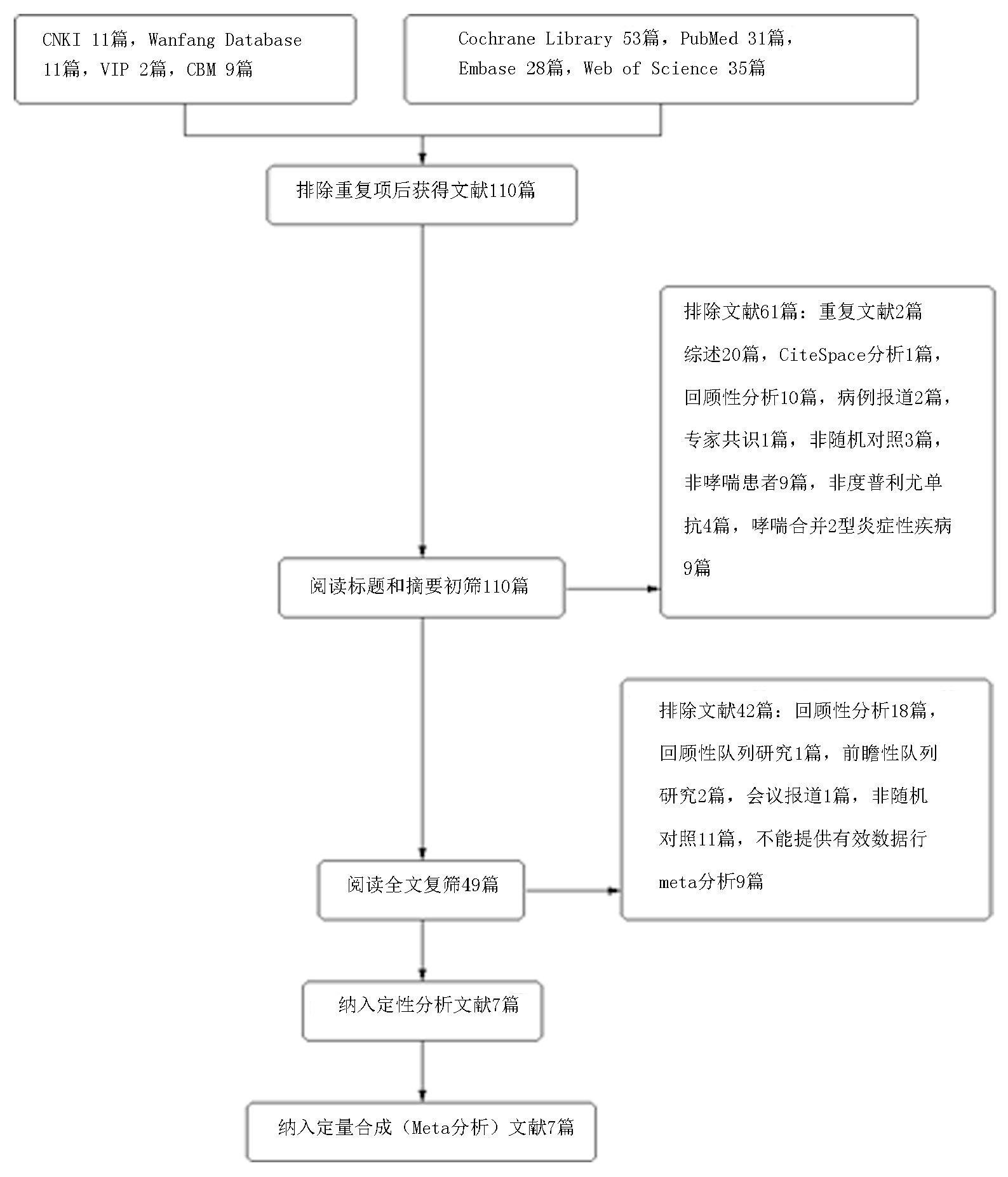
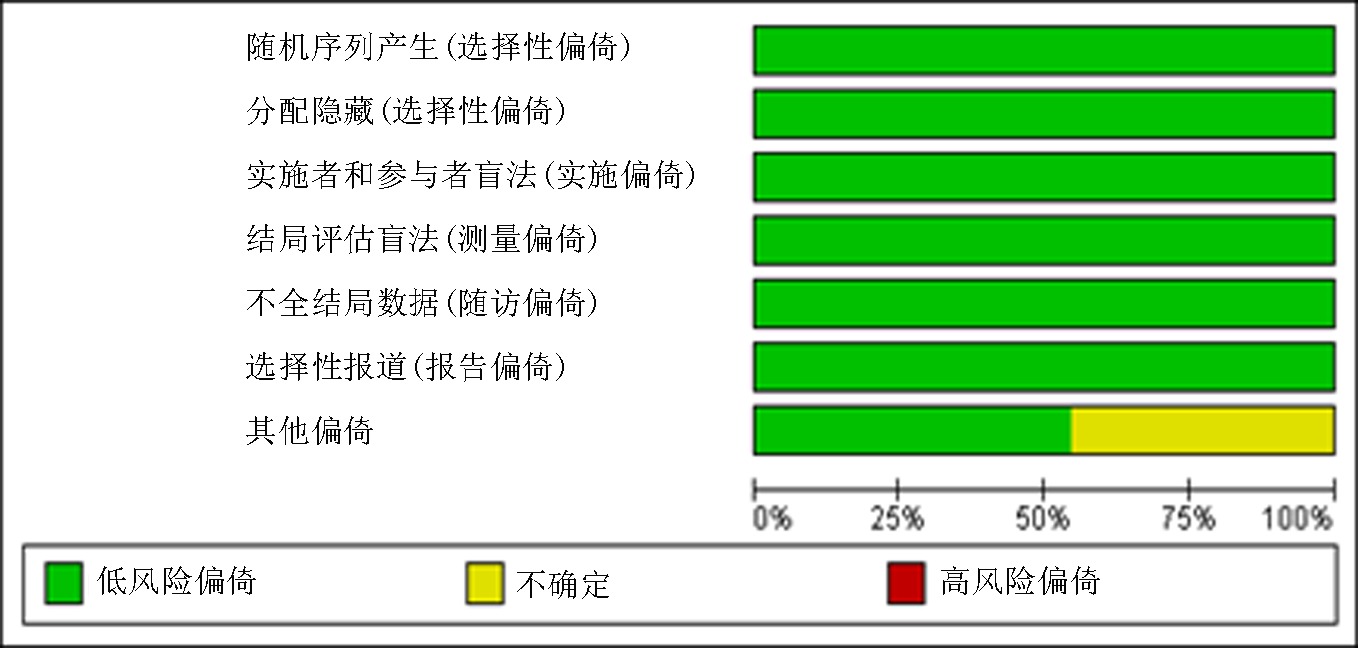
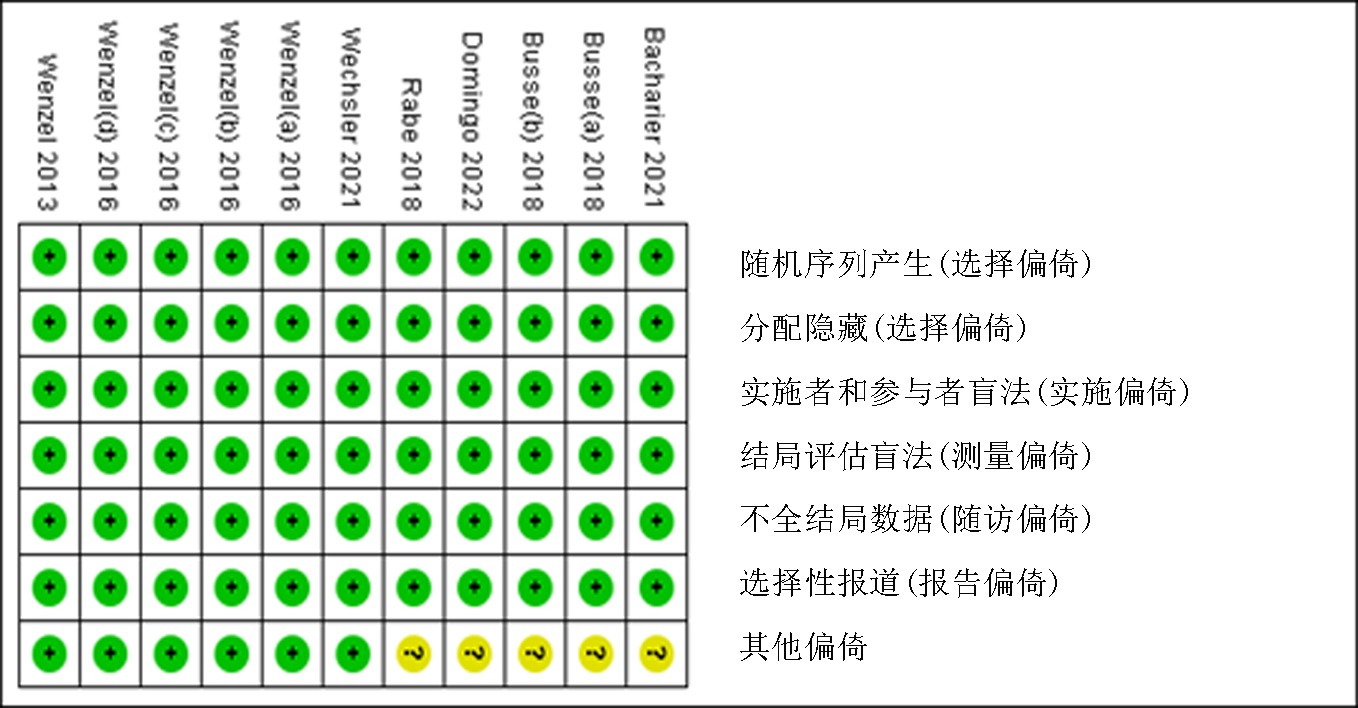
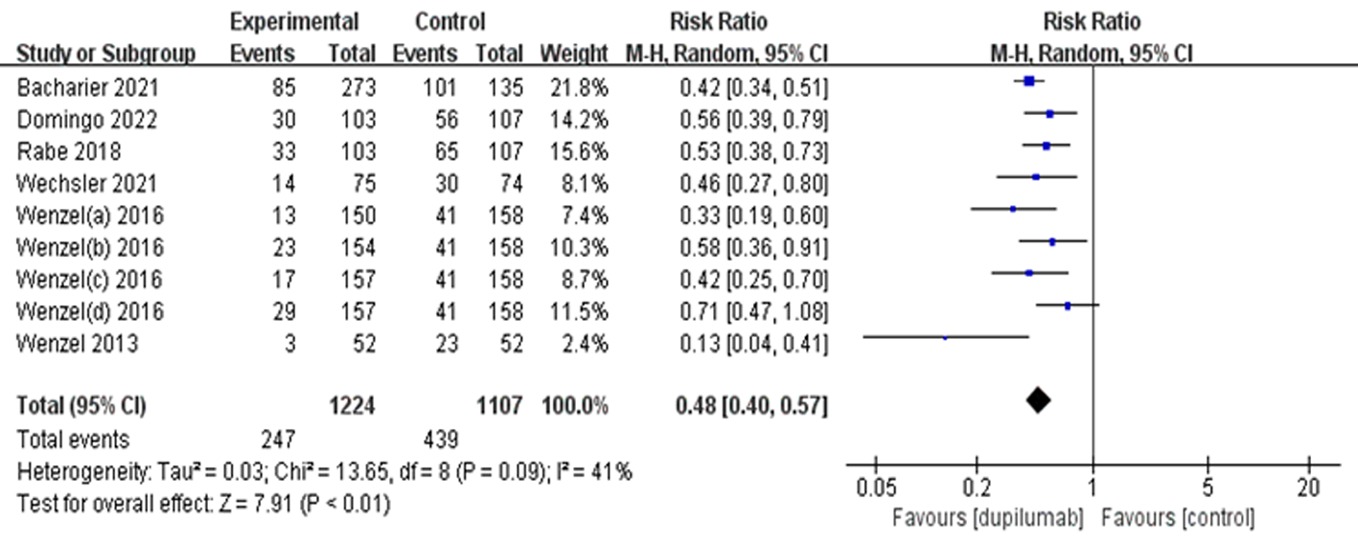
Clinical Focus ›› 2023, Vol. 38 ›› Issue (10): 869-877.doi: 10.3969/j.issn.1004-583X.2023.10.001
Meta-analysis of the efficacy and safety of dupilumab on the treatment of bronchial asthma
Ruan Junwen1( ), Zhou Jianrong2, Liu Weiyou2, Yuan Xiaoliang2, Yan Hao3
), Zhou Jianrong2, Liu Weiyou2, Yuan Xiaoliang2, Yan Hao3
- 1. The First Clinical Medical College, Gannan Medical University, Ganzhou 341000,China
2. Department of Respiratory and Critical Care Medicine, First Affiliated Hospital of Gannan Medical University,Ganzhou 341000,China
3. Clinical Medical College, Jiangxi University of Chinese Medicine,Nanchang 330004, China
-
Received:2023-04-20Online:2023-10-20Published:2024-01-03 -
Contact:Ruan Junwen E-mail:ruanjunwen2020@163.com
CLC Number:
Cite this article
Ruan Junwen, Zhou Jianrong, Liu Weiyou, Yuan Xiaoliang, Yan Hao. Meta-analysis of the efficacy and safety of dupilumab on the treatment of bronchial asthma[J]. Clinical Focus, 2023, 38(10): 869-877.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://huicui.hebmu.edu.cn/EN/10.3969/j.issn.1004-583X.2023.10.001
Tab.1 Basic characteristics of included studies
| 纳入研究 | 病例数 | 平均年龄(岁) | 干预措施 | 疗程 | 结局指标 | ||||
|---|---|---|---|---|---|---|---|---|---|
| T/C | T | C | T | C | |||||
| Bacharier 2021[ | 273/135 | 8.9±1.6 | 9.0±1.6 | 达必妥100 mg q2w(W≤30kg), 200 mg q2w(W>30kg) | 安慰剂q2w | 52w | ①②③④⑥⑦ | ||
| Busse(a) 2018[ | 634/317 | ≥12 | 达必妥200 mg q2w | 安慰剂q2w | 52w | ② | |||
| Busse(b) 2018[ | 634/317 | ≥12 | 达必妥300 mg q2w | 安慰剂q2w | 52w | ② | |||
| Domingo 2022[ | 103/107 | 52.9±12.6 | 50.3±12.6 | 达必妥300 mg q2w | 安慰剂q2w | 24w | ①②④⑥ | ||
| Rabe 2018[ | 103/107 | 51.9±12.5 | 50.7±12.8 | 达必妥300 mg q2w | 安慰剂q2w | 24w | ①②③⑥⑦ | ||
| Wechsler 2021[ | 75/74 | 51.3±12.7 | 47.0±11.4 | 达必妥300 mg q2w | 安慰剂q2w | 12w | ①②③④⑤⑥⑦ | ||
| Wenzel(a) 2016[ | 150/158 | 48.6±13.0 | 达必妥200 mg q2w | 安慰剂q2w | 24w | ①②④⑤⑥⑦ | |||
| Wenzel(b) 2016[ | 154/158 | 48.6±13.0 | 达必妥200 mg q4w | 安慰剂q4w | 24w | ①②④⑤⑥⑦ | |||
| Wenzel(c) 2016[ | 157/158 | 48.6±13.0 | 达必妥300 mg q2w | 安慰剂q2w | 24w | ①②④⑤⑥⑦ | |||
| Wenzel(d) 2016[ | 157/158 | 48.6±13.0 | 达必妥300 mg q4w | 安慰剂q4w | 24w | ①②④⑤⑥⑦ | |||
| Wenzel 2013[ | 52/52 | 37.8±13.2 | 41.6±13.1 | 达必妥300 mg qw | 安慰剂qw | 12w | ①④⑥⑦ | ||
Tab.1 Basic characteristics of included studies
| 纳入研究 | 病例数 | 平均年龄(岁) | 干预措施 | 疗程 | 结局指标 | ||||
|---|---|---|---|---|---|---|---|---|---|
| T/C | T | C | T | C | |||||
| Bacharier 2021[ | 273/135 | 8.9±1.6 | 9.0±1.6 | 达必妥100 mg q2w(W≤30kg), 200 mg q2w(W>30kg) | 安慰剂q2w | 52w | ①②③④⑥⑦ | ||
| Busse(a) 2018[ | 634/317 | ≥12 | 达必妥200 mg q2w | 安慰剂q2w | 52w | ② | |||
| Busse(b) 2018[ | 634/317 | ≥12 | 达必妥300 mg q2w | 安慰剂q2w | 52w | ② | |||
| Domingo 2022[ | 103/107 | 52.9±12.6 | 50.3±12.6 | 达必妥300 mg q2w | 安慰剂q2w | 24w | ①②④⑥ | ||
| Rabe 2018[ | 103/107 | 51.9±12.5 | 50.7±12.8 | 达必妥300 mg q2w | 安慰剂q2w | 24w | ①②③⑥⑦ | ||
| Wechsler 2021[ | 75/74 | 51.3±12.7 | 47.0±11.4 | 达必妥300 mg q2w | 安慰剂q2w | 12w | ①②③④⑤⑥⑦ | ||
| Wenzel(a) 2016[ | 150/158 | 48.6±13.0 | 达必妥200 mg q2w | 安慰剂q2w | 24w | ①②④⑤⑥⑦ | |||
| Wenzel(b) 2016[ | 154/158 | 48.6±13.0 | 达必妥200 mg q4w | 安慰剂q4w | 24w | ①②④⑤⑥⑦ | |||
| Wenzel(c) 2016[ | 157/158 | 48.6±13.0 | 达必妥300 mg q2w | 安慰剂q2w | 24w | ①②④⑤⑥⑦ | |||
| Wenzel(d) 2016[ | 157/158 | 48.6±13.0 | 达必妥300 mg q4w | 安慰剂q4w | 24w | ①②④⑤⑥⑦ | |||
| Wenzel 2013[ | 52/52 | 37.8±13.2 | 41.6±13.1 | 达必妥300 mg qw | 安慰剂qw | 12w | ①④⑥⑦ | ||
| [1] | Global Initiative for Asthma. 2021 GINA report, global strategy for asthma management and prevention(2021 update)[EB/OL].https://ginasthma.org/gina-reports. |
| [2] | Allan R, Canham K, Wallace R, et al. Usability and robustness of the wixela inhub dry powder inhaler[J]. Aerosol Med Pμlm Drug Deliv, 2021, 34(2):134-145. |
| [3] |
Huang K, Yang T, Xu J, et al. Prevalence, risk factors and management of asthma in China: A national cross-sectional study[J]. Lancet, 2019, 394(10196):407-418.
doi: S0140-6736(19)31147-X pmid: 31230828 |
| [4] |
Song WJ, Kang MG, Chang YS, et al. Epidemiology of adμlt asthma in Asia: Toward a better understanding[J]. Asia Pac Allergy, 2014, 4(2):75-85.
doi: 10.5415/apallergy.2014.4.2.75 URL |
| [5] |
Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma[J]. Eur Respir J, 2014, 43(2):343-373.
doi: 10.1183/09031936.00202013 pmid: 24337046 |
| [6] |
Settipane RA, Kreindler JL, Chung Y, et al. Evaluating direct costs and productivity losses of patients with asthma receiving GINA 4/5 therapy in the United States[J]. Ann Allergy Asthma Immunol, 2019, 123(6):564-572.
doi: 10.1016/j.anai.2019.08.462 URL |
| [7] |
Rudolph AK, Walter T, Erkel G. The fungal metabolite cyclonerodiol inhibits IL-4/IL-13 induced Stat6-signaling through blocking the association of Stat6 with p38, ERK1/2 and p300[J]. Int Immunopharmacol, 2018, 65:392-401.
doi: 10.1016/j.intimp.2018.10.033 URL |
| [8] |
Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation[J]. Allergy, 2020, 75(5):1188-1204.
doi: 10.1111/all.v75.5 URL |
| [9] |
Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma[J]. N Engl J Med, 2018, 378(26):2475-2485.
doi: 10.1056/NEJMoa1804093 URL |
| [10] | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1, The Cochrane Collaboration, 2011[EB/OL].http://www.cochrane-handbook.org. |
| [11] |
Bacharier LB, Maspero JF, Katelaris CH, et al. Dupilumab in children with uncontrolled moderate-to-severe asthma[J]. N Engl J Med, 2021, 385(24):2230-2240.
doi: 10.1056/NEJMoa2106567 URL |
| [12] |
Busse WW, Maspero JF, Rabe KF, et al. Liberty asthma QUEST: Phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma[J]. Adv Ther, 2018, 35(5):737-748.
doi: 10.1007/s12325-018-0702-4 pmid: 29725983 |
| [13] |
Domingo C, Maspero JF, Castro M, et al. Dupilumab efficacy in steroid-dependent severe asthma by baseline oral corticosteroid dose[J]. J Allergy Clin Immunol Pract, 2022, 10(7):1835-1843.
doi: 10.1016/j.jaip.2022.03.020 pmid: 35398549 |
| [14] |
Wechsler ME, Ruddy MK, Pavord ID, et al. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma[J]. N Engl J Med, 2021, 385(18):1656-1668.
doi: 10.1056/NEJMoa2024257 URL |
| [15] |
Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial[J]. Lancet, 2016, 388(10039):31-44.
doi: 10.1016/S0140-6736(16)30307-5 pmid: 27130691 |
| [16] |
Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels[J]. N Engl J Med, 2013, 368(26):2455-2466.
doi: 10.1056/NEJMoa1304048 URL |
| [17] |
Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma[J]. Am J Respir Crit Care Med, 2009, 180 (5):388-395.
doi: 10.1164/rccm.200903-0392OC URL |
| [18] |
Frøssing L, Silberbrandt A, Von Bülow A, et al. The prevalence of subtypes of type 2 inflammation in an unselected population of patients with severe asthma[J]. J Allergy Clin Immunol Pract, 2021, 9 (3):1267-1275.
doi: 10.1016/j.jaip.2020.09.051 pmid: 33039645 |
| [19] |
Chuang YT, Leung K, Chang YJ, et al. A natural killer T-cell subset that protects against airway hyperreactivity[J]. Allergy Clin Immunol, 2019, 143(2):565-576.
doi: 10.1016/j.jaci.2018.03.022 URL |
| [20] |
Eger K, Kroes JA, Brinke AT, et al. Long-Term Therapy Response to Anti-IL-5 Biologics in Severe Asthma-A Real-Life Evaluation[J]. J Allergy Clin Immunol Pract, 2021, 9(3):1194-1200.
doi: 10.1016/j.jaip.2020.10.010 pmid: 33069885 |
| [21] |
Riccio AM, Dal Negro RW, Micheletto L, et al. Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients[J]. Int J Immunopathol Pharmacol, 2012, 25(2):475-484.
doi: 10.1177/039463201202500217 pmid: 22697079 |
| [22] |
Nair P, Pizzichini MMM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia[J]. N Engl J Med, 2009, 360(10):985-993.
doi: 10.1056/NEJMoa0805435 URL |
| [23] |
Casale TB, Luskin AT, Busse W, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study[J]. J Allergy Clin Immunol Pract, 2019, 7(1):156-164.
doi: S2213-2198(18)30323-4 pmid: 29800752 |
| [24] |
Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moder-ate-to-severe uncontrolled asthma[J]. N Engl J Med, 2018, 378 (26):2486-2496.
doi: 10.1056/NEJMoa1804092 URL |
| [25] | Castro M, Rabe KF, Corren J, et al. Dupilumab improves lung function in patients with uncontrolled, moderate-to-severe asthma[J]. ERJ Open Res, 2020, 6 (1):00204-2019. |
| [26] |
Dupin C, Belhadi D, Guilleminault L, et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort[J]. Clin Exp Allergy, 2020, 50 (7):789-798.
doi: 10.1111/cea.13614 pmid: 32469092 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||